Guiding decision making with safety and efficiency in mind
Detect cognitive changes precisely and quickly without burdening participants or raters

Validated, proprietary electronic cognitive assessments to determine cognition in everyday life activities.
Quick and easy to administer, these proprietary, digital scales can be used throughout your study to identify subtle changes in executive function, cognition and everyday life activities in all participant populations.
Designed by WCG therapeutic area experts, these scales guide decision making with safety and efficiency in mind, providing investigators with meaningful results while reducing rater and participant burden.
Learn more about WCG’s proprietary scales and how they can be used to support the unique needs of your study and your participants.
WCG’s Proprietary Rater Scales
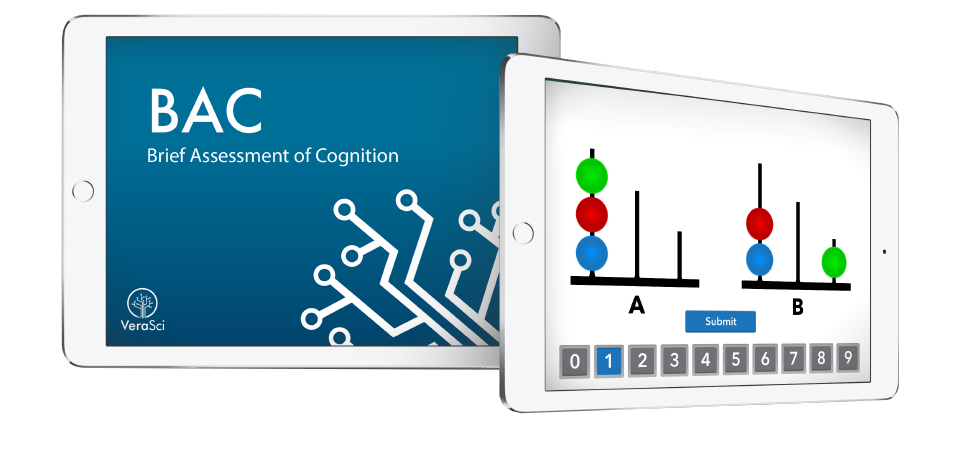
BAC
Our proprietary Brief Assessment of Cognition (BAC) is a user-friendly electronic cognitive assessment battery with improved sensitivity to impairment.
VRFCAT
Our proprietary Virtual Reality Functional Capacity Assessment Tool (VRFCAT) integrates virtual reality into the assessment of functioning in clinical trials.
SCoRS
Our proprietary Schizophrenia Cognition Rating Scale (SCoRS) is an interview-based clinical measure sensitive to treatment effects.
SNAPSI
Our Simplified Negative and Positive Symptoms Interview facilitates quick and valid rating on the 6-item version of the Positive and Negative Syndrome Scale.
Learn more about WCG’s proprietary assessments
Brief Assessment of Cognition (BAC)
BAC is a user-friendly electronic cognitive assessment battery with improved sensitivity to impairment.
BAC is quick and easy to administer while preserving the human interaction of traditional neurocognitive testing that is necessary for impaired populations. The electronic BAC Assessment provides investigators with meaningful results while reducing rater and participant burden.
The tablet-based Brief Assessment of Cognition in Schizophrenia is a proprietary measure designed by WCG experts.
The BAC Measures Performance Across Multiple Cognitive Domains:
- Verbal Memory
- Processing Speed
- Working Memory
- Verbal Fluency
- Motor Function
- Executive Function
Key benefits of the BAC:
- Scientifically validated
- Large database of available normative data
- Alternate forms for repeated testing
- Automated response capture and scoring
- High reliability and sensitivity to impairment
- Available in multiple languages
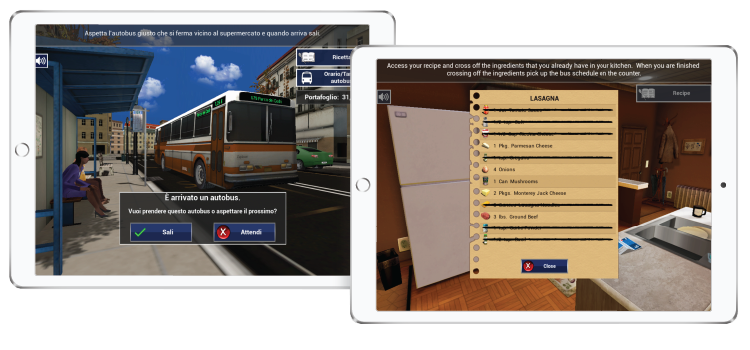
The Virtual Reality Functional Capacity Assessment Tool (VRFCAT)
The VRFCAT is a tablet-based task that simulates key instrumental activities of daily living (iADLS) in a realistic and interactive virtual environment.
With demonstrated sensitivity to basic functional capacity deficits, the VRFCAT was developed to improve clinical trials by detecting functionally meaningful improvements in participants’ everyday lives.
The VRFCAT has numerous advantages over conventional assessments, and meets the highest psychometric and regulatory standards, with strong support from industry sponsors, NIH and FDA as a functional co-primary outcome measure.
The VRFCAT has officially been accepted into the FDA’s COA Qualification Program as a measure of functional capacity for schizophrenia treatment trials. This FDA program is intended to qualify drug development tools that can be relied on to have a specific interpretation and application in any drug development program and regulatory review. The FDA also awarded a grant to conduct further research on the VRFCAT.
Advantages of the VRFCAT:
- Scientifically validated
- Accurate, performance-based results
- Integrated data capture
- Alternate forms for repeated testing
- Eases administrator burden
- Well-tolerated by participants
- Available in multiple languages and culturally adapted environments
- Fast, responsive 24/7 technical support
- FDA 21 CFR Part 11 Compliant
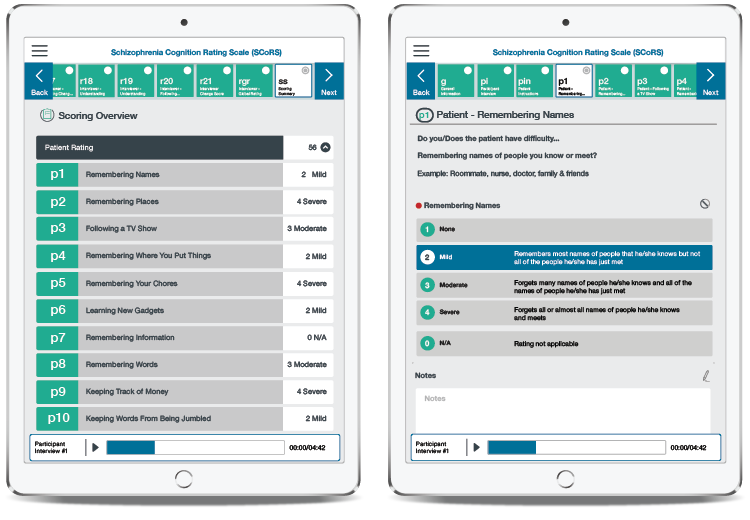
Schizophrenia Cognition Rating Scale (SCoRS)
The Schizophrenia Cognition Rating Scale (SCoRS) is a 20-item interview-based clinical assessment available on tablet or via pen-and-paper that evaluates cognitive deficits and the degree to which these deficits impair participants’ day-to-day functioning. It was originally developed in 2001 at Duke University Medical Center by Dr. Richard Keefe and is licensed through WCG. The SCoRS is used in registration-level clinical trials, academic research and in clinical settings.
The SCoRS contains questions about the participant’s ability to manage cognitively demanding, functionally relevant, everyday tasks such as conversations, watching television and using electronic devices.
The items were developed to assess the following cognitive domains:
- Attention
- Memory
- Working Memory
- Language Production
- Reasoning
- Problem Solving
- Motor Skills
- Social Cognition
As an interview-based assessment of cognition, the SCoRS meets the criteria established by the FDA-NIMH-MATRICS panel for co-primary outcome measures for cognitive enhancement trials in schizophrenia. Contact us today to learn more about the Schizophrenia Cognition Rating Scale.
The best way to predict the likelihood of your research study is to determine it
There’s no time for doubt or delays. WCG’s clinical endpoint solutions demystify trial efficacy by reducing clinical trial error rate and, subsequently, the risk of inconclusive and unsalvageable studies.