Site Enablement for Sponsors & CROs
Connecting sponsors and CROs with advanced solutions to help optimize their study sites for faster clinical trials.

Accelerate your trials and reach your study endpoints faster with our site enablement solutions.
Complex protocols, increased demands for data collection, and ongoing pressure to achieve study endpoints faster are putting increased burdens on clinical trial teams. Each trial has its own concerns, and understanding each study’s unique characteristics is critical to identifying the areas that commonly contribute to study delays.
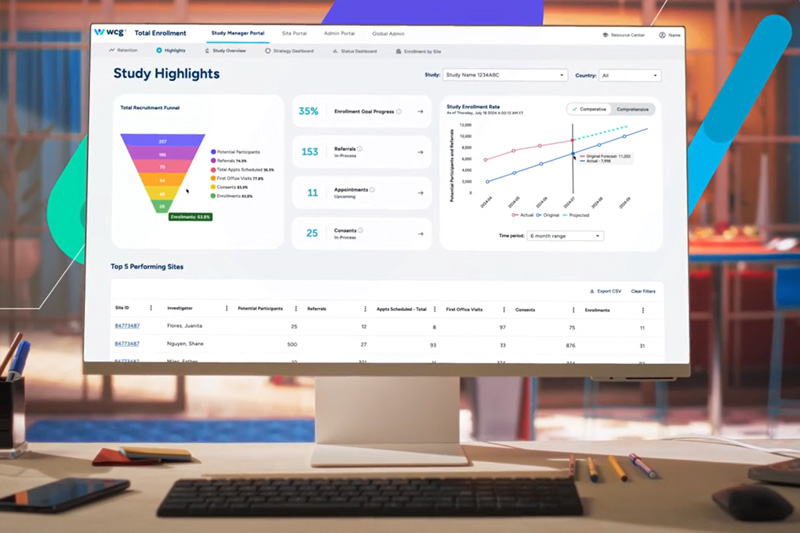
To enable studies to reach endpoints faster, WCG offers a set of comprehensive and integrated solutions to accelerate research activities at sites and conduct your trials more efficiently. Powered by advanced technology, scientific and operational expertise, and our proprietary healthcare data set, WCG Data Intelligence, our Site Enablement solutions are designed to identify study bottlenecks, activate sites faster, and accelerate enrollment.
For starting and conducting your studies as efficiently as possible, WCG offers
WCG Site Network
Participant Identification
Participant Enrollment
Participant Retention
Documentation Support
Clinical Trial Training
Safety Letter Acknowledgement
Rater Qualification and Training
Regulatory Document Exchange
Technologies that support the success of your studies and sites
Accelerate your trials and reach your study endpoints faster.
Complete the form to schedule a consultation with WCG.
Recent insights

Delve into the World of Psychedelic Research and Ethical Inquiry
Podcasts
A 360-degree View on Motivators and Deterrents to Clinical Trial Participation
Podcasts