Protect your Clinical Trial Endpoints
Reduce the risk of clinical trial failure with continuous, scientific protection against threats to your study endpoints.

Clinical trial validity: An ongoing concern
The COVID-19 pandemic fundamentally disrupted the clinical trial landscape. Studies shifted from physical sites to virtual environments, and many studies are still virtual. Each clinical trial has its own protocol-design challenges, which are amplified when trials are conducted virtually.
Accordingly, operational changes must be informed by the scientific implications, not the other way around. This requires a unique, scientific, and comprehensive solution aimed at assessing and protecting study endpoints.
Clinical expertise, scientific knowledge, trusted solutions
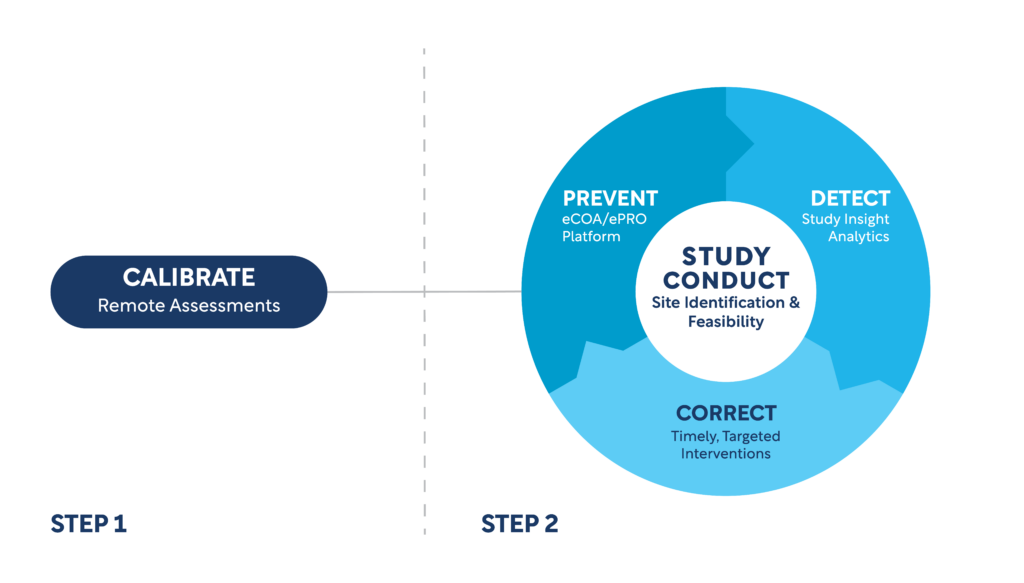
WCG has a purpose-built solution to continuously calibrate, prevent, detect, and correct threats to study endpoints.
Remote Assessments
Collect data remotely or on-site with HIPAA- and GDPR-compliant audio/video technology.
WCG’s Remote Assessment system minimizes data variability and allows sponsors to maintain scientifically valid data.
eCOA/ePRO Platform
Establish and maintain the quality of your participant- and clinician-reported outcomes.
WCG’s evidence-based platform integrates clinical and scientific expertise with adapted technology to reduce error rates for study endpoints.
Study Insight Analytics
Pinpoint and analyze anomalies in clinical trial data collected remotely.
WCG’s Study Insight Analytics combine statistical process control and other analytical techniques with real-time human expertise. This approach provides insights and actionable recommendations, allowing sponsors to address data-quality issues and protect study outcomes.
Threat Detection
Deploy comprehensive training and mitigation steps as soon as errors and anomalies are identified.
WCG’s portfolio of tailored, evidence-based interventions mitigates the risks of bias, variability, and human error in clinical trials, particularly those with subjective endpoints. The result: better caliber of data, clearer signals, and enhanced study success rates.
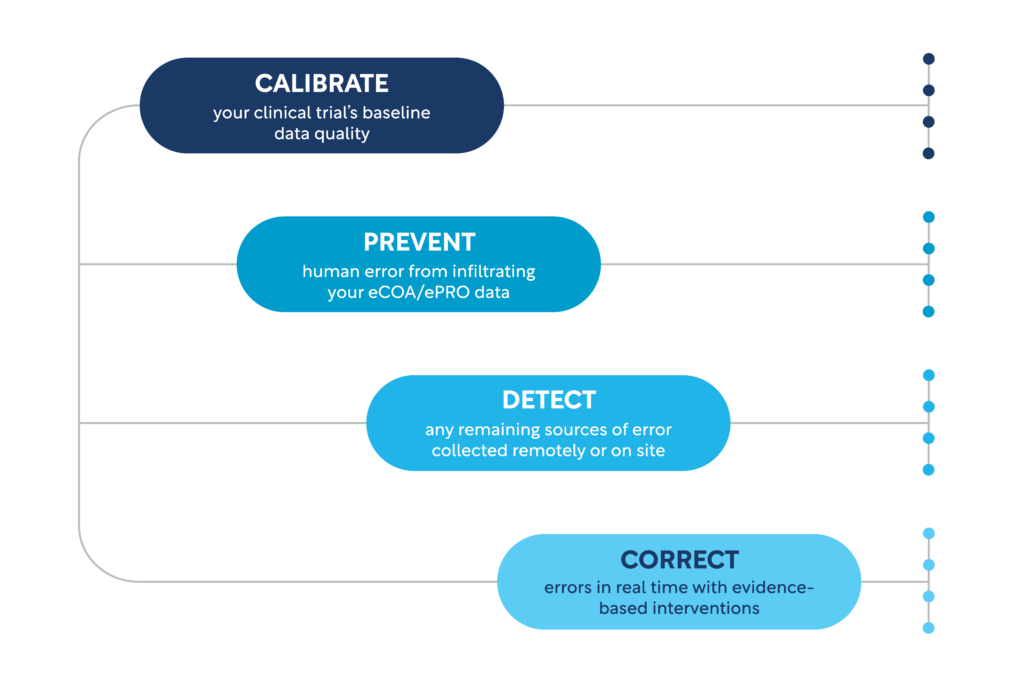
4 Layers of Defense Against Increased Variability & Measurement Error
Though decentralized and hybrid trials are propelling study conduct forward, technology-based solutions alone are not enough to defend against increased variability and measurement error. Scientific validity and operational feasibility must be ingrained in every aspect of a clinical trial.
Continuously Protect Your Study Endpoints
By continuously protecting primary and subjective endpoints, sponsors can rest assured that the variability of their clinical trial data will be thoroughly minimized. Whether you’re pursuing a traditional, remote, or hybrid clinical trial, our purpose-built solution addresses the most significant threats to sponsors’ clinical endpoints.

A Roadmap to Success
Our Endpoint Protection solution gives you the tools to:
- Establish the baseline quality of your participant- and clinician-reported data
- Virtualize your scales with tailored, scientific rigor
- Implement a detection mechanism to pinpoint study outliers
- Clinically assess and analyze incoming data
- Deploy targeted interventions, informed by actionable insight.
For more than 25 years, in over 500 global studies, WCG has instilled the highest scientific validity and operational excellence into every program. Each service is tailored to minimize data variability, thereby reducing the risk of inconclusive, unsalvageable studies.
Contact us to discuss how you can quickly deploy Endpoint Protection for your studies
Our team is ready to discuss how we can help you achieve your goals for your studies. Complete this form to request a consultation.