eResearch eXpress CTMS
Enhance Your Research Site’s Efficiency with Centralized Study Management, Improved Financial Oversight, and Streamlined Workflows.
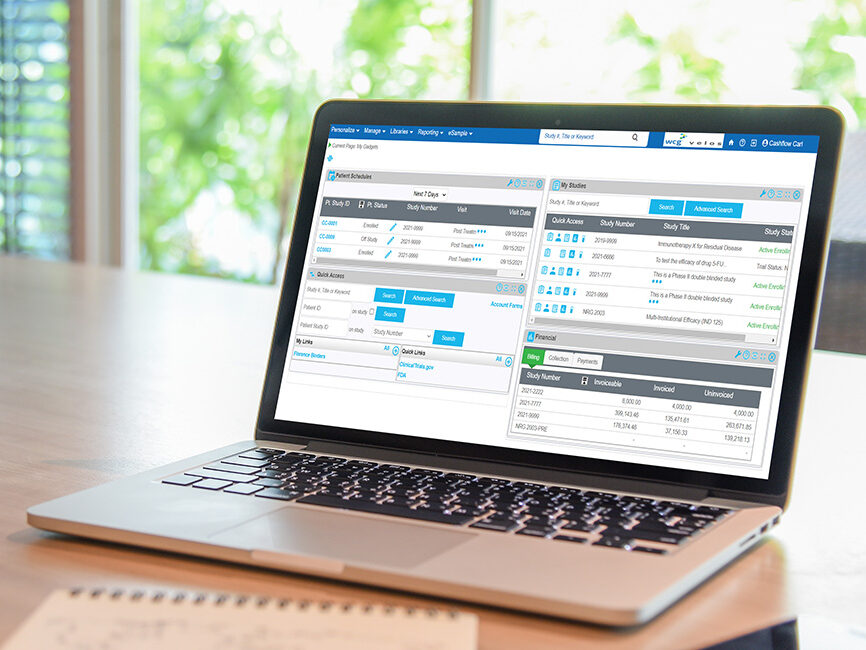
WCG eResearch eXpress is a ready-to-use, pre-configured, cloud-based Clinical Trial Management System (CTMS) that centralizes your site’s research operations, bringing financial, operational, and regulatory activities together in a unified, automated solution.
This affordable, accessible, and scalable technology is made to empower research sites, hospitals, and health systems of all sizes to efficiently manage and streamline their research programs through a SaaS system. WCG eResearch eXpress can be implemented in less than a week, providing a rapid return on investment (ROI) for your site.
Say goodbye to manual processes and unreliable spreadsheet tracking; WCG eResearch eXpress offers an intuitive, compliant solution that enhances efficiency, oversight, and profitability for sites.
Why Choose WCG eResearch eXpress CTMS?
WCG eResearch eXpress CTMS was designed with sites in mind. It’s the perfect CTMS for sites looking to streamline their research operations and enhance transparency through a ready-to-go SaaS tool.
Ready-to-Use SAAS Technology
Experience seamless set-up with WCG eResearch eXpress’s pre-configured CTMS, enabling you to be fully operational within days. Our SaaS technology allows you to have the latest features and updates as they are available.
Increased Efficiency and Financial Oversight
Streamline research management processes and reduce the administrative burden on your site staff while gaining tools for better financial management.
Rapid Implementation and Personalized Training
Gain a best-in-class CTMS without the wait: eResearch eXpress can be implemented in less than one week. Our expert team will provide training and support so you can get up and running quickly.
Accessible Price Point and Minimal IT Requirements
Enjoy budget-friendly pricing and minimal IT requirements, ensuring advanced clinical trial management is accessible to sites of all sizes.
Fully Compliant
Meet industry standards effortlessly with a 21 CFR Part 11 and HIPAA-compliant system, providing data integrity and security.
Data Migration Options
Transition from other CTMS platforms, paper, and Excel with our extensive experience in migrating data.
Key Features of eResearch eXpress CTMS
Study Initiation
Study Management
Study Closeout

Study Setup
- Protocol Definition
- Study Calendar
- Budget
- Coverage Analysis
- CRF Design
- Study Team
- Status Tracking

Participant
Management
- Coordinator Portal
- Participant Profile
- Participant Status
- Consent
- Visit Tracking
- Deviation Tracking
- Participant Portal
Financial
Management
- Charge Master
- Milestones
- AR/AP
- Invoicing
- Payment
- Reconciliation
Clinical Data
Management
- Electronic Data Capture
- Case Report Forms (eCRFs)
- Data Monitoring
- Query Management

Reporting &
Dashboards
- Standard Dynamic Reports
- Ad-Hoc Queries
- Study Dashboards
- Finance Dashboards
- Data Monitoring Dashboards
- Query Management Dashboards
eResearch eXpress CTMS FAQs
- Site admin access and login information for eResearch eXpress are provided at the onboarding meeting.
- A general walkthrough of the technology is provided at the onboarding meeting, and access is granted to other site users.
- The site logo and Charge Master are uploaded into the site’s eResearch eXpress CTMS environment.
- Live virtual training is scheduled and provided by the dedicated eResearch eXpress CTMS training team.
- A session with an eResearch eXpress CTMS subject matter expert is scheduled to provide guidance on best practices for using the technology.
- Weekly check-ins are scheduled for the first six weeks of implementation.
WCG eResearch eXpress CTMS can be implemented in less than a week, providing a rapid return on investment (ROI) for your site.
Yes! WCG eResearch eXpress CTMS is a 21 CFR Part 11 and HIPAA-compliant system, ensuring your site meets industry standards while providing you with data integrity and security.
Yes, our team has extensive experience migrating data from other CTMS platforms, paper documents, and Excel into eResearch eXpress CTMS.
Schedule an eResearch eXpress CTMS Demo
Learn how you can simplify clinical research at your site, optimize your research program, and improve productivity. Complete this form to request a demo of our industry-leading technology.
Recent Insights

The 2025 Avoca State of the Industry Report: Understanding Adoption & Implementation of ICH E6(R3)
Reports
Decentralized Trials: Thoughts for Sponsors, Investigators, and IRBs
Blog Posts
Compounded Drugs in Research: Navigating IND Exemptions and Current Regulations
Blog Posts