
Lindsay McNair, MD, MPH, MSB
Chief Medical Officer, WCG (Former)
Biography
Dr. Lindsay is the former Chief Medical Officer for WCG. She oversaw the physician team within the WCG IRBs, and provided consultation to institutions and pharma/biotech companies on a wide range of issues related to protocol design, regulatory compliance, human subject protection, and ethical policy development (pre-approval access, subject compensation).
Prior to joining WCG, Dr. McNair was a consultant to multiple biopharma companies, providing medical guidance on clinical development strategies and study designs for new drug studies, and medical oversight of all phases of clinical trials. Before becoming a consultant, Dr. McNair was the medical lead for the telaprevir development program at Vertex Pharmaceuticals, with oversight of the phase 1-3 studies.
Dr. McNair is adjunct faculty at Boston University and teaches graduate courses on the scientific design of clinical research studies.
Dr. McNair graduated from the University of Connecticut School of Medicine and trained in general surgery at Boston University Medical Center. She completed her Master’s in Public Health at Boston University concentrating in Biostatistics/Epidemiology, and her Master’s of Science in Bioethics at Union Graduate College concentrating in research ethics.
Dr. McNair is an associate editor for the Journal of Empirical Research on Human Research Ethics, and serves on multiple committees including the NYU Compassionate Use Pre-Approval Access (CUPA) Working Group, the Human Subjects Review Board of the US Environmental Protection Agency (EPA HSRB), and the Advancing Effective Research Ethics Oversight (AEREO) consortium.
Latest insights by Lindsay

Is IRB review required when linking to clinicaltrials.gov postings on patient advocacy websites and newsletters?
Blog Posts
COVID-19 Insights from Former FDA Commissioner, Scott Gottlieb, MD
Blog Posts
Can participants be reconsented over the phone when an ICF is updated?
Blog Posts
Virtual Clinical Trials: Best Practices in Moving Toward a Patient-Centric Research Model
Whitepapers
Is IRB review required for survey results that may be published?
Blog Posts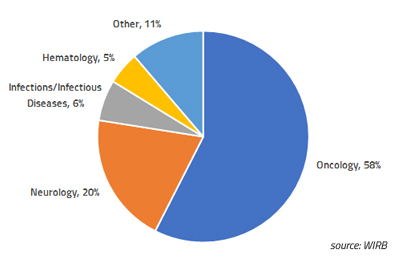
Single-Patient Expanded Access: WIRB experience in 2018
Blog Posts
Recommendations for Study Sponsors on Informed Consent Documents
Whitepapers
Looking Ahead in 2019 Whitepaper
Whitepapers
Returning Study Results to Research Participants
Whitepapers