WCG’s IRB+ Streamlines Ethical Review for Faster Study Start-Up
Premium Attention. Personalized Oversight. Accelerated Timelines.
Do you have a study that needs an extra level of attention and white-glove service to ensure accelerated processing times without sacrificing the quality or integrity of the Institutional Review Board (IRB)?
WCG’s IRB+ service provides a fully-dedicated team, working seven days per week overseeing your study throughout the entire operational process.
Realize the benefits of a 50% reduction in turnaround times, with WCG’s innovative approach to document processing. This premium service can shave months off your protocol review, leading to significantly faster study activation.
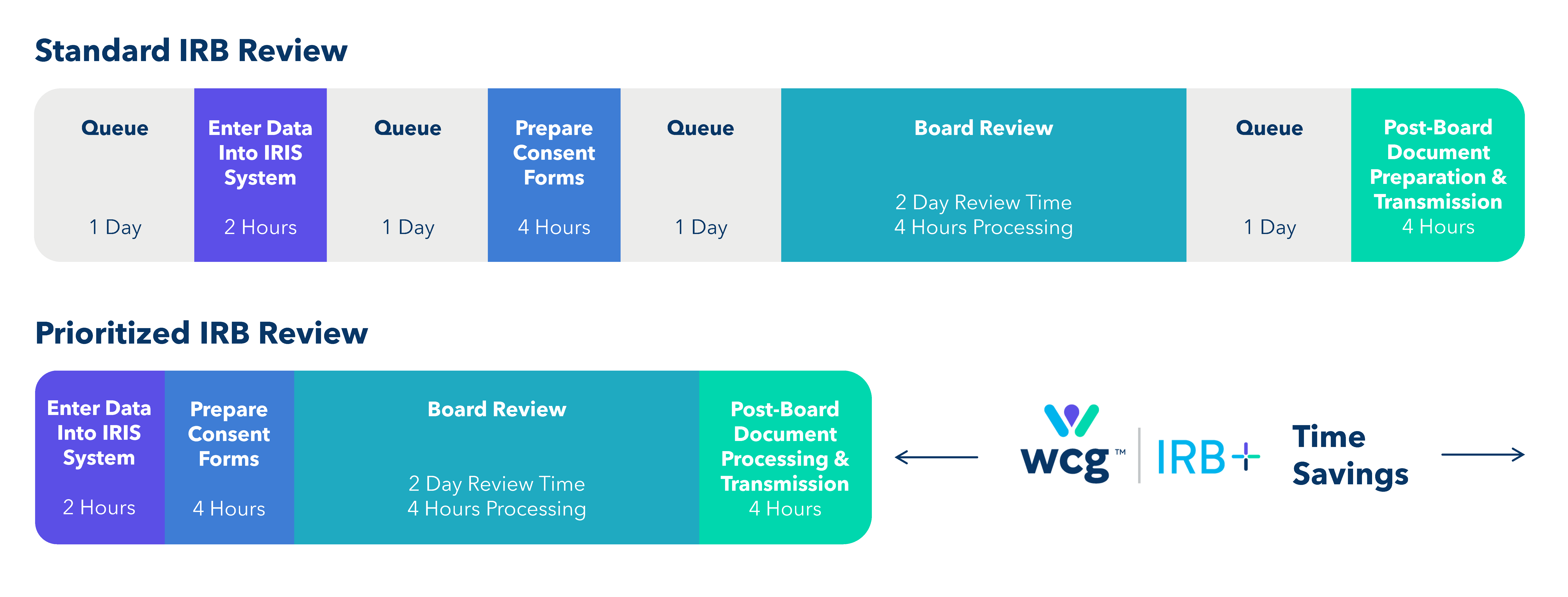
Reinventing Turnaround Times for IRB Review
A Dedicated Team
Studies submitted via IRB+ are backed by a dedicated team of 17 operational experts and 300+ support personnel.
7 Day Operational Workweek
WCG’s IRB+ operations teams work 12 hours per day, seven days per week processing your documents to ensure the fastest timelines for your submission to be reviewed. We are working, even when you aren’t!
Save Time & Maintain Quality
Quicker reviews mean faster study starts. WCG’s IRB+ provides up to a 50% reduction in turnaround times. This can equate to months saved on site activation and study start-up.
Realize the Benefits of a Combined IRB+ and IBC+ Review Process
Does your study involve a genetically modified product and require Institutional Biosafety Committee (IBC) review? Increase your time savings and reduce burden on your sites by combining WCG’s IBC+ and WCG’s IRB+. Learn more about IBC+ today.
Stop waiting for your trial to start.
WCG has reinvented the IRB operational process to provide the fastest turnaround without sacrificing the sanctity of ethical review. Experience the WCG difference starting with a free IRB+ review consultation. We’re here to help you streamline your clinical trial processes.