Clinical research is always about the participant. We all agree on that, but how often is that participant’s perspective considered throughout study planning? It can get lost in the complexity and activity of study startup.
Sites and sponsors must begin with one question: What makes sense for the participant?
One of the first things we can do to understand the participant is to build a participant profile. That requires insight into all five Ws:
- Who is volunteering to participate? Who else may be involved (e.g., family members, children, caregivers, etc.)? Will there be any long visits or overnight stays? Think about the ways being part of this study will interfere with the participant’s lives.
- Where is the study taking place?Is the location convenient for the participant? If not, how will they get there? People may travel hours each way when the site is a larger medical center. Is that practical for frequent visits? “Where” isn’t just about physical access: Where are these individuals relative to the site? Are we looking for participants already part of the site’s healthcare system? If not, is the site in network with their insurance? Will there be potential costs for the participants?
- When does the study begin, and how long will it last? Are there seasonal factors to consider? Do the study visits work with school and work schedules? Can they align with regular checkups?
- Why do they want to be in the study? For example,is it altruism? Monetary reward? Is it an opportunity to a receive novel treatment otherwise unavailable?
- What happens if the individual doesn’t enroll? Are there other treatment options? Will they have the opportunity to reconsider?
These factors will be different for each site, each study, and each participant, so this list is simply a starting point, not a blueprint.
The Participant Journey
Answering those questions informs the participant journey. That journey should move clearly from point A to point B to point C. Figure 1 illustrates this process. Without that pathway in place, it will be difficult to plan how you will recruit, enroll and retain study participants.
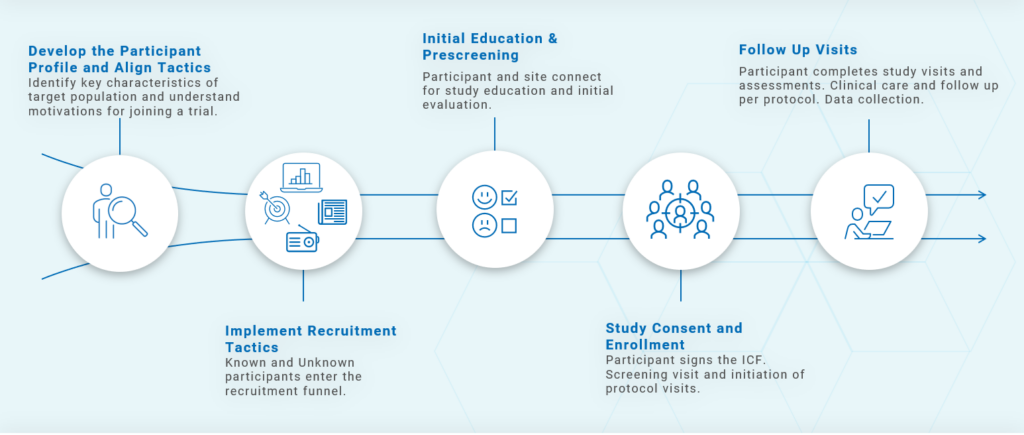
- Develop the participant profile: Build the profile based on the questions discussed above.Understand what motivates and what worries the participant. If we are able to tap into that, we can engage the participant not only during recruitment and enrollment, but throughout the entirety of the trial.
- Develop recruitment tactics that align with the profile. Decide how you will reach new participants. Will you go out into the community? Use vendor resources? Turn to social media or advertising? Determining your recruitment tactics depends on how you answer the five Ws above.
- Provide initial education and prescreening: It’s just as important for the study to be right for the participant as it is for the participant to be right for the study. Participants need to fully understand what’s involved. And yes, after they do, many may decide against moving forward with enrollment, but learning that as soon as possible is best for the individual and your study.
- Study consent and enrollment: Carefully explain the Informed Consent form (ICF). Never send the ICF to someone without thoroughly reviewing it with them in advance. An ICF can be scary, and you don’t want it to be the reason you lose a potential enrollee.
- Clinical care: Once you enroll the participant, the real work begins. From Adverse Event (AE) monitoring and data entry to follow-up care and the final study visit, it’s essential to ensure the participant journey continues smoothly and ends successfully.
All this comes back to tailoring efforts around what works for the potential participant, the specific study, and each site.
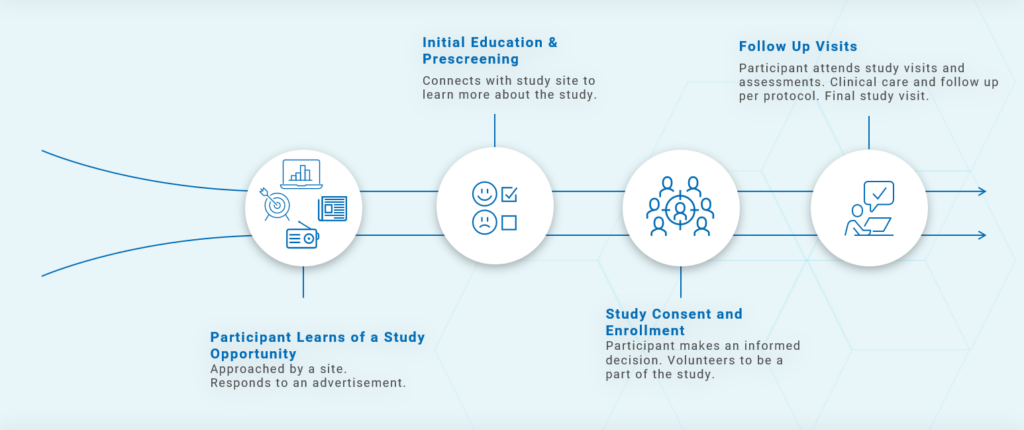
Finally, keep in mind that this process isn’t the one participants see. From their perspective, the journey is (or should be) much simpler, as illustrated in Figure 2.
Communication across stakeholders
Effective communication supports successful recruitment and retention: Everyone knows what they’re expected to do. Such transparency supports collaboration and creates accountability.
Newsletters, webinars and study coordinator meetings can keep sites, investigators and sponsors grounded and on the same page. Most important is having a centralized tracking platform that sites and sponsors can view such as WCG’s My Patient. Transparency and easily accessible information can reduce the back and forth among sites and sponsors to keep the focus on the participants.
To enhance communications with participants:
- Keep them apprised of updates, timeline adjustments, protocol amendments and other changes.
- Provide after-hours support. Stay in touch: For instance, making a brief reminder call the day before a visit could be the difference between someone showing up or missing the appointment.
- Give each participant a point of contact. This enhances communication and shows you care about the participant. Along those same lines, show appreciation. After all, they didn’t have to enroll. Taking the extra five minutes to send a thank you card, or text shows your gratitude and helps keep them engaged between visits.
A Proven Strategy
When we focus on the participant’s perspective, we improve recruitment and retention and increase the likelihood of a successful trial. But successfully implementing a participant-centered approach requires sites and sponsors to use and understand the resources and processes that support open communication with participants.
For example, WCG supported a vaccine study in an elderly population seen in Figure 3. During the development stage of the enrollment strategy, we focused on tailoring all content to be very specific to the participant population of the study, which included understanding what other possible comorbidities and health treatments the participants may likely be experiencing. The information provided to the participants was targeted, clear and easily digestible. But simply creating customized content was only the beginning. We knew that engaging in continuous communication throughout the participant journey was the key to success.
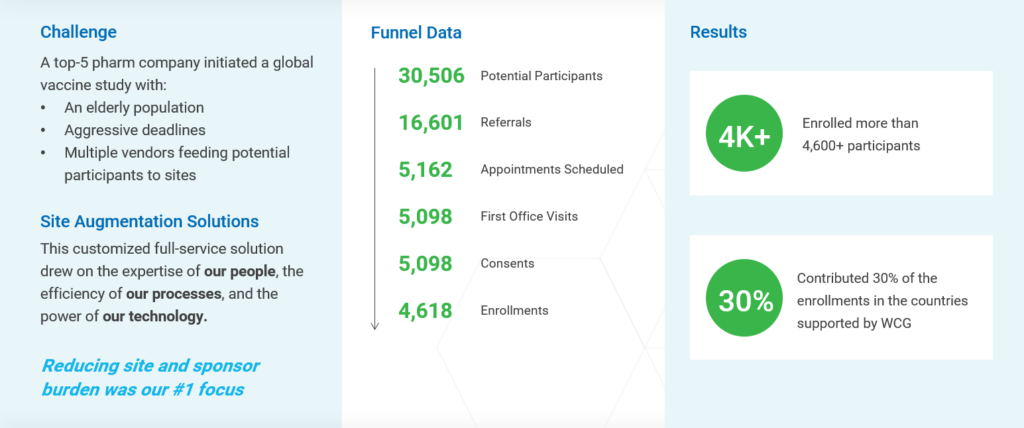
With keeping the participant as the central focus of our process, we were able to establish as a 98% conversion rate between appointment scheduled and first office visit attendance. Additionally, every participant who attended the first visit also consented into the study.
This isn’t just theory: We’ve guided hundreds of sites and sponsors, helping them implement a participant-centered approach that boosts recruitment, enrollment, and retention. If you’d like to learn more, fill out the form below to meet with our experts.
Make recruitment and retention easier for your clinical trial
Complete the form to schedule complimentary consultation with our experts today. We’ll help assess your need, and discuss how WCG can assist.
