Historically, clinical trials of therapeutic outcomes in arthritis have primarily relied on patient questionnaires for assessing progression of disease, focused on clinical parameters including pain, quality of life, and joint function. Sole reliance on clinical parameters has several limitations however, including the inherent subjectivity involved in the assessment of patient experience, as well as the necessity that a patient exhibit overt symptoms. Some therapeutic interventions aim to slow or halt progression of cartilage loss before the patient becomes symptomatic, significantly decreasing the utility of patient questionnaires as a trial endpoint.
Imaging Related Endpoints
Clinical efficacy trials in orthopedics rely on clinical, laboratory, and imaging outcome measurements in order to assess disease activity and progression. Often, the radiographic imaging measurements are one of the trials primary endpoints. This is particularly evident in trials evaluating therapeutic interventions in patients with rheumatoid arthritis or osteoarthritis, as well as in post- operative patients after the placement of spinal fusion implants, fracture fixation devices, and joint reconstruction hardware. The role of the imaging core laboratory in these trials primarily involves the performance of an independent analysis and reviews of the radiographic images, using either a semi-quantitative or fully quantitative approach, in order to assess the efficacy of the therapeutic intervention. The use of imaging related endpoints in orthopedic clinical trials, in addition to clinical assessments and patient feedback, is important precisely because of this ability to compile quantitative data.
Historically, clinical trials of therapeutic outcomes in arthritis have primarily relied on patient questionnaires for assessing progression of disease, focused on clinical parameters including pain, quality of life, and joint function. Sole reliance on clinical parameters has several limitations however, including the inherent subjectivity involved in the assessment of patient experience, as well as the necessity that a patient exhibit overt symptoms. Some therapeutic interventions aim to slow or halt progression of cartilage loss before the patient becomes symptomatic, significantly decreasing the utility of patient questionnaires as a trial endpoint.
Osteoarthritis and Rheumatoid Arthritis
In the setting of osteoarthritis and rheumatoid arthritis, assessment of structural damage to the articular cartilage has been one of the primary means of monitoring the progression of the disease, and in the case of pharmacologic interventions, assessing the efficacy of a given therapy. This assessment has, to date, primarily been performed with serial radiographic monitoring as the primary imaging endpoint. This necessitates that the serial radiographs be performed with a standardized, reproducible, validated technique, with independent review, interpretation, and analysis—hence the importance of the imaging core laboratory in these clinical trials.
In order to achieve these results, quantitative imaging analysis has primarily entailed measuring of joint space width (JSW) and joint space narrowing (JSN) on serial radiographs in arthritic patients. Despite the ability of these measurements to provide quantitative, relatively objective data, the sensitivity of these measurements for progression of osteoarthritis remains low, and large patient samples as well as serial assessment over several years are typically necessary for a given trial. Furthermore, measurements of joint space width and joint space narrowing are inherently flawed because they are measurements of negative space—measurements of what is not visualized between adjacent bones, such as the femoral condyle and the tibial plateau in the setting of knee arthritis. While cartilage loss certainly contributes to this negative space, other morphologic abnormalities including deformity of subchondral bone and loss of meniscal tissue compromise the validity of these measurements.
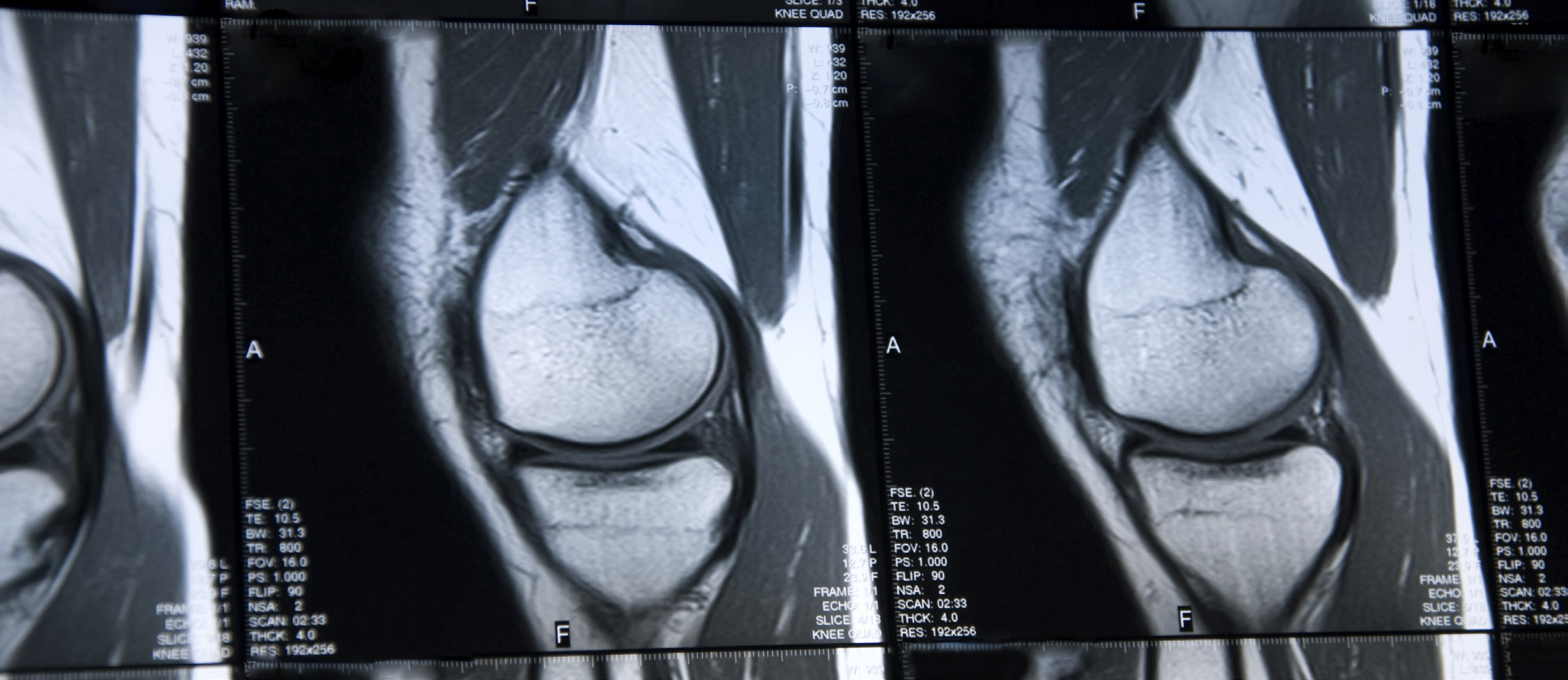
Quantitative Magnetic Resonance Imaging
Quantitative magnetic resonance imaging (MRI) has begun to emerge as a viable imaging endpoint in orthopedic trials. Unlike arthroscopy, which is invasive and evaluates the surface and thickness of the cartilage, and radiography, which provides a two dimensional measurement of negative joint space as discussed above, MRI has the ability to directly visualize the entire thickness and volume of the cartilage. Both morphologic changes and structural integrity can be assessed, and three dimensional volumetric measurements performed with the appropriate software. Using either a 1.5 or 3.0 Tesla scanner, dedicated evaluation of cartilage volume and morphologic characteristics can be performed using one or more of several sequences, including, but not limited to, fat-saturated intermediate- weighted (proton density weighted) fast spin-echo images, T1-weighted 3D high-spatial-resolution volumetric fat- suppressed spoiled gradient-echo (SPGR), multi echo data image combination (MEDIC) images, or dual-echo steady- state (DESS) images. Evaluation of the cartilage collagen network and proteoglycan content can be performed with alternative techniques, including T2 mapping, delayed gadolinium-enhanced MR imaging of cartilage (dGEMRIC), T1 imaging, sodium imaging, and diffusion weighted imaging. These techniques can provide detailed evaluation of cartilage integrity, structural composition, and volume in clinical trials assessing the benefits of pharmacologic interventions. However, by directly visualizing the morphology and composition of the cartilage, as well as the integrity of the underlying subchondral bone and surrounding soft tissues, these techniques also offer the potential for semiquantitative and quantitative evaluation of patients after surgical intervention. This would include both cartilage reparative techniques, such as microfracture, as well as reconstructive procedures such as autologous chondrocyte implantation.
Given the ability of MRI to visualize the adjacent soft tissues, as opposed to standard radiography, MRI has now also become a primary outcomes endpoint in rheumatoid arthritis phase 2 clinical trials—crucial in assessing the severity and progression of disease in these patients. The superior soft tissue contrast of MRI, allowing evaluation of subchondral erosions and synovial and bony hyperemia, inaddition to the morphologic integrityof cartilage, coupled with a formalized Rheumatoid Arthritis Scoring in Magnetic Resonance Imaging (RAMRIS) system, allows for earlier evaluation of pharmacologic interventions such as disease-modifying anti-rheumatic drug (DMARD) therapy. This increased sensitivity to early joint inflammation and destruction, before radiographic changes are apparent, streamlines and shortens the decision making process on whether a given drug will proceed to phase 3 clinical trials.
Novel MRI techniques have also opened the door to earlier evaluation of complications after the placement of orthopedic hardware, particularly joint arthroplasty, with the superior soft tissue contrast of MRI allowing for the earlier assessment of particle disease and metallosis. Small particles of polyethylene, metal, or cement shed from the arthroplasty incite, in a subset of patients, a localized inflammatory response resulting in effusion, thickening of the synovial envelope, and eventually osteolysis. In the case of metal-on-metal implants, typically composed of cobalt-chrome, metal ions are released both in the joint as well as systemically, and incite perivascular lymphocyte and plasma cell infiltrates around the prosthesis—termed aseptic lymphocytic vasculitis-associated lesions (ALVALs). MRI is used to assess for the associated presence of periarticular fluid collections or masses, termed pseudotumors. The lining of these pseudotumors is contiguous with the joint capsule, so that they actually represent localized expansion of the synovial envelope with fluid and metallic debris. Early detection of these soft tissue manifestations of particle disease is vital to postoperative monitoring of these patients, with identification of particle disease before the disease has progressed to gross failure of the prosthesis. Quantitative MRI assessment as an imaging endpoint is then possible, with the ability to assess the disease burden of these soft tissue manifestations of particle disease with two- dimensional or three-dimensional volumetric measurements.
Summary
Previously the utility of MRI in arthroplasty imaging was limited, due to the magnetic susceptibility of the hardware, which distorted the regional magnetic field and significantly degraded image quality immediately surrounding the implant. Novel techniques including MARS (metal artifact reduction sequence), MAVRIC (multi-acquisition variable- resonance image contribution), and SEMAC (slice encoding for metal artifact correction) employ a wide receiver bandwidth and maximization of gradients to reduce frequency shifts that otherwise distort MR images around the implant. Additional advances include the use of high resolution matrices in the frequency encoding direction, as well as the use of fast inversion recovery sequences (STIR), and avoidance of gradient echo and spectral fat suppression techniques. Both changes in the composition of implants (titanium is now widely used, which is less ferromagnetic than cobalt chromium), as well as these novel MRI techniques, have opened the door for MRI as an imaging tool in the evaluation of orthopedic hardware—in the clinical as well as in the research setting.
Given these applications of quantitative MRI in the monitoring of disease progression in osteoarthritis and rheumatoid arthritis, as well as new and expanding applications in prosthesis and orthopedic hardware evaluation, MRI now has an increasing role as a primary imaging endpoint in orthopedic clinical trials.
Volume 5, Issue 11: Guidance for Sponsors: Quantitative MRI Imaging in Orthopedic Clinical Trials
Originally written by legacy Intrinsic Imaging Medical Director
Contact WCG Imaging to discuss your trial’s imaging needs
We have the team, therapeutic expertise, technology, and ISO-certified quality management systems to provide imaging core lab services to our clients worldwide. Complete the form to get started.