This is the first of a two-part blog series of how to create more efficiency in your clinical trial training efforts using hybrid training approaches that can save time while reducing complexity and redundancy in clinical trial training.
Training. A crucial step in the critical path of study start-up, and on paper, a seemingly straightforward one. But somewhere along the way, training becomes complex, making it easy to lose focus of what really matters to ensure a trial is conducted properly. Many teams are overwhelmed by the complexity of training and the resulting impact on how investigators, clinicians and study teams engage, learn and stay informed.
All too often, we encounter missed opportunities for training. The PI’s flight is delayed to the live investigator meeting. A clinician with a busy daily practice can’t make time for a webinar. Good Clinical Practices (GCP) training requirements seem beyond absurd. There is seemingly constant turnover on study teams and sites often join the study late.
A recipe for complexity …or is it?
All of the above are true and do add complexity — but a better way to simplify this resides in adopting a hybrid approach (combination of in-person and on-demand training) to training investigators and study teams. Here are five powerful questions you can ask when considering a hybrid approach.
1. What is the REAL focus of the Investigator Meeting?
Quite often, we place a burden on the in-person Investigator Meeting with the long list of objectives it must satisfy. The IM is a chance to, with limited distraction, review the protocol, understand the science at a deeper level and most importantly, collaborate with investigator teams’ exclusive peer groups to discuss everything from new ideas and solutions to practical site-specific questions. As WCG conducts over 100 live investigator meetings each year, we understand that a well-executed IM can deliver on each of those objectives, but all too often, more requirements of the IM are piled on (including GCP training) which encroaches on the schedule at the IM. The key insight? The IM is a critical opportunity for training – but in one moment in time – where all time is a premium.
2. How can I create more time at the Investigator Meeting for what really matters?
This is a helpful question allowing thought about how preparation and using a hybrid approach to training can simplify the process. Things like pre-work completed before the IM, GCP training that can be accessed through an on-demand clinical training library prior to the meeting. Specific modules that are pre-requisites or require more time to convey can also be taken before the IM using an on-demand platform — on investigators own time, when it’s convenient for them, anywhere it’s convenient for them. Even SIV and amendment concerns can be covered at the IM with a plan in mind, leveraging the power of one platform for closing training and amendment gaps.
3. What are the benefits of a hybrid approach to training?
While there are many reasons to embrace a hybrid approach to training, the best may be that it makes the lives of investigators and study teams easier and better. More specifically, it allows for more focus and less distraction. This kind of focus leads to better understanding of what is different about the protocol, inclusion/exclusion specifics and how to be successful on THIS particular study. It frees up time for more discussion of the details that could be difference-maker for success.
4. How can a hybrid approach manage the unknown obstacles that inevitably occur with training?
A hybrid approach delivers flexibility, transparency and helps avoid redundancy of training. The flexibility to conduct pre-work or ICH-GCP investigator training that focuses on investigators responsibilities as outlined in section 4 of the guideline allows this to be completed in advance and on-demand to the availability of the investigator. A platform that supports both GCP and protocol-specific training also provides a roadmap of how to train when the inevitable issues arise. Redundant training can also be avoided as certification and compliance directories can cross check for training already completed that does not require repeat.
5. Can this approach save both time and resources?
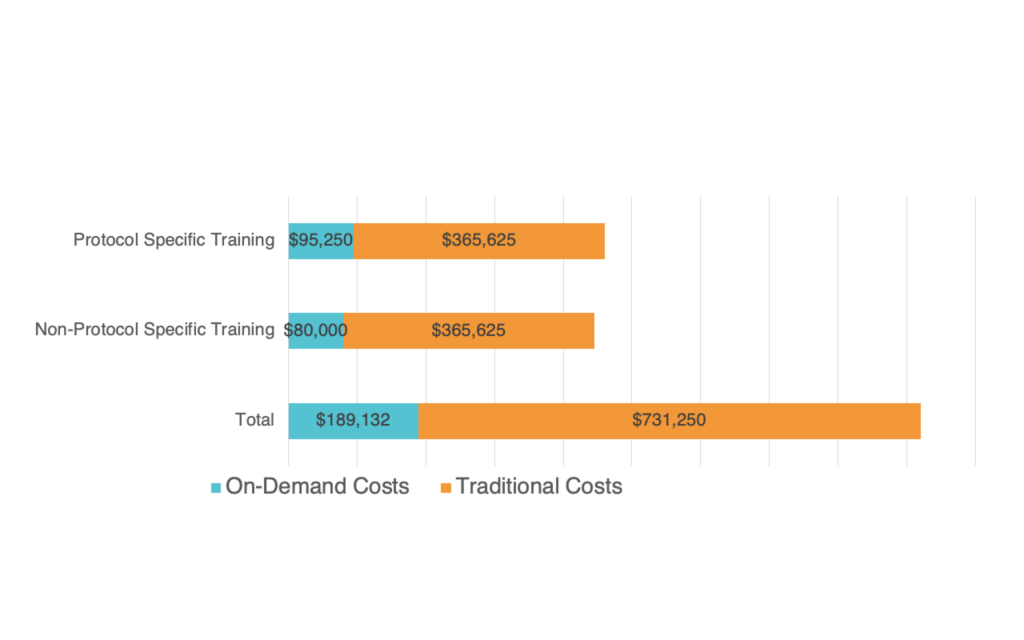
This approach can reduce the number of days (or even hours) required for the IM. This time is valuable from both a productivity standpoint and a cost savings standpoint. It is estimated that cost savings using on-demand training are significant, with on-demand delivering ~75% cost advantage over traditional solutions.
Below, we compare and contrast the potential cost savings on-demand training delivers over traditional investigator meeting solutions. Assumptions are based on 150 sites (2 trainees per site) and 25 participants from Sponsor/CRO and three global face-to-face meetings.