The notice of proposed rulemaking (NPRM) for Cooperative Research, or the single Institutional Review Board (sIRB) requirement by the Food and Drug Administration (FDA), was issued in the Federal Register in September 2022. The NPRM was issued in response to the legislative mandate under the 21st Century Cures Act for the FDA to harmonize its regulations as much as possible with the 2018 revisions to the Common Rule and changes to Cooperative Research under 45 CFR 46.114. The public comment period for the NPRM closed in November of 2022. Once the final rule goes into effect, all US institutions participating in multi-site cooperative research regulated by the FDA will need to rely on approval by a single IRB unless certain proposed exceptions are met under the regulation. Since exceptions are anticipated to be rare, this change will impact most FDA-regulated research.
The single IRB mandate will require that many institutions that do not wish or lack the capacity to act as single IRBs rely on other central IRBs. Although the goal of the single IRB mandate is to decrease administrative burdens at the institutional and investigator level, the transition will result in changes in institutional policies and structures. Sponsors will be required to use a single IRB. Institutions or investigators will need to use the single IRB chosen by the sponsor or choose not to participate in the research. Many institutions are putting processes in place now, with anticipation that the final rule will be issued soon. The FDA has noted in its Unified Agenda that the final rule is expected to be issued in January 2026, but this date is not guaranteed and is subject to change.
Why Does It Take so Long for Regulations to be Issued by the FDA and Other Federal Agencies?
Rulemaking is a complex process that is outlined under the Administrative Procedures Act (5 USC §553 (1946)). The Act requires that an NPRM be published in the federal register to allow for public comments unless the agency issuing the rule finds the notice and public comments to be impracticable, unnecessary, or not in the public interest. Public comments generally close after 60 days of publication of the federal register notice. In cases where it is determined that an NPRM is not needed, an interim or final rule may be issued, depending on the specific situation. Once the comment period has closed, FDA staff must then go through the process of reviewing those comments and deciding if points raised in the comments require modification of the proposed rule. Any revisions will require consensus among the stakeholders involved in writing the rule. In addition to this, there are many internal agency and interagency steps that occur from the time rule making is initiated until a final rule is published, further lengthening the timeline.
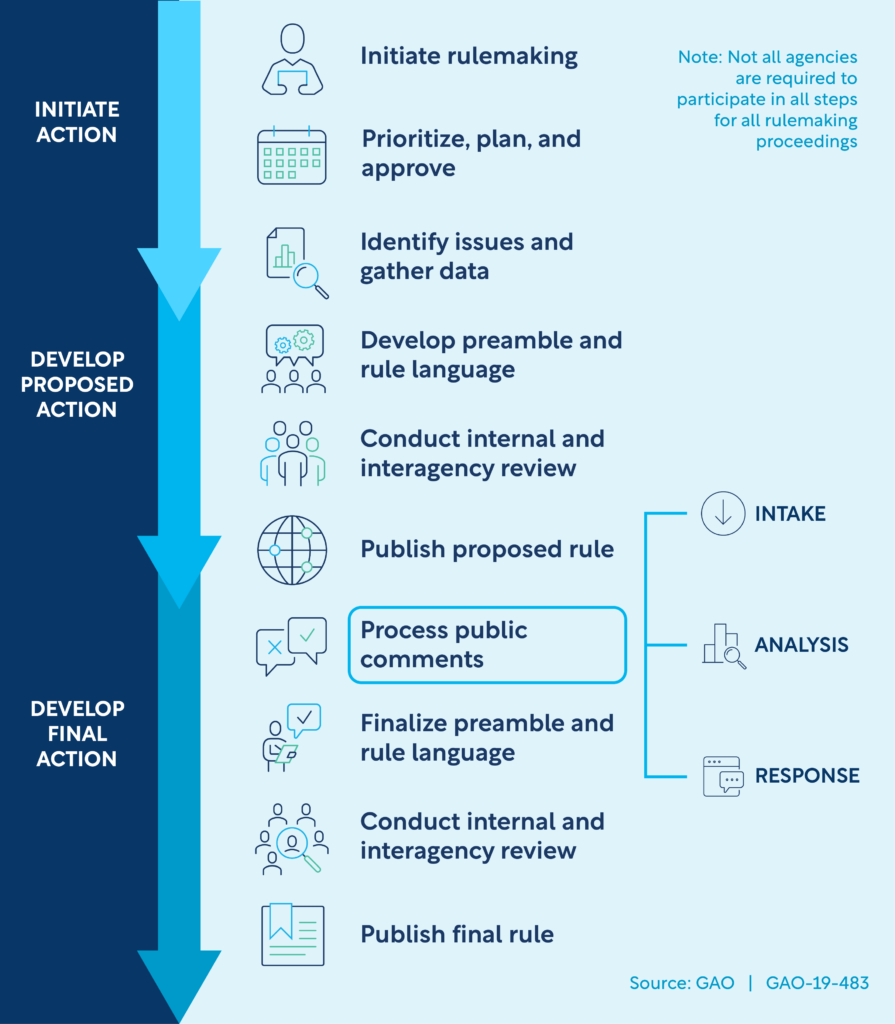
For FDA review, many offices can be involved in the process of rulemaking. Relevant offices such as the Office of Clinical Policy, Office of Medical Policy, Office of the Chief Counsel, and the Office of the Commissioner might be involved in developing the rule. Other offices, depending on the topic of the rule may also be engaged. For example, for topics related to human subject protection, the Office of Clinical Policy is generally included in the discussions, or if the topic is pediatric, the Office of Pediatric Therapeutics may be engaged. Often working groups are created that include the appropriate office and subject matter experts and these groups meet regularly. However, in some cases competing priorities may disrupt the meeting schedule and some time may lapse between meetings.
Once a draft rule is created by a working group, the proposed rule circulates through various levels of review at the FDA. If significant changes are made as part of the review process by one office, re-review may be necessary to ensure agreement. This is the most time-consuming part, because some offices may need to re-review several times if certain aspects of their input are revised by other groups. Each office needs sufficient time to review, which may take several weeks within each office. Once draft language is agreed upon, a formal clearance process takes place. This may include additional offices and stakeholders that may not have been involved initially. Again, adequate time for review is needed by each office and stakeholder. At the FDA, certain agency priorities may slow the process. For example, the COVID-19 pandemic directed agency resources to develop guidance for COVID-19-related issues and away from ongoing routine processes. If legislation is enacted that requires guidance to be written within specific timelines, the attention from the work on rulemaking may be redirected to handle these time-sensitive projects.
Once FDA clearance is complete, interagency review occurs. The more complex or impactful the rule, the more likely that several agencies will need to review it. Although those agencies may not be disclosed, for the single IRB rule, review by the Department of Health and Human Services (HHS), as well as the Office of Human Research Protections (OHRP) would certainly need to take place given the impact on the existing regulations on cooperative research under the Common Rule.
When a final rule is issued, the FDA is likely to allow a grace period for implementation. However, whether a grace period is allowed and/or the length of any grace period is not stipulated in the NPRM. Regarding the single IRB mandate, some may have processes in place for single IRB review for NIH-supported research. If not, it would be prudent for sponsors and institutions to consider any changes in processes that might need to be made now, so that they are ready when the rule goes into effect.
Consult the Experts to Prepare for the Single IRB
WCG has unmatched expertise in supporting all stakeholders in clinical research with navigating changes so your organization remains compliant and efficient. Leverage the industry-leading IRB review vendor by completing the form below.
Don't trust your study to just anyone.
And we’re the best for a reason. Experience the WCG difference starting with a free ethical review consultation. We’re here to help you streamline, alleviate, and accelerate.
