Gastroenterology

Regulatory Compliance
Unpacking the FDA’s Guidance on Developing Drugs for Treatment in Pediatric Inflammatory Bowel Disease
Blog Posts
Site Efficiency
WCG’s 2024 Clinical Research Site Challenges Report
Whitepapers
Participant Recruitment
WCG Contributed 30% of Study Enrollments While Enabling Timely Participant Data Entry
Case Studies
Participant Recruitment
800+ Pre-Qualified Participants Transferred to Study Sites for IBD Portfolio
Case Studies
WCG Revolutionizes Ethical Review Process with IRB+ Service, Achieving Unprecedented Efficiency Gains
News
Participant Recruitment
WCG Increased Enrollment by 67%
Case Studies
Series: WCG Talks Trials
Amplifying Patient Voices to Advance Clinical Research: The Importance of Patient Advocacy
Podcasts
Diversity & Inclusion
The Importance of Diversity, Equity, Inclusion, and Intersectionality in Clinical Research
Podcasts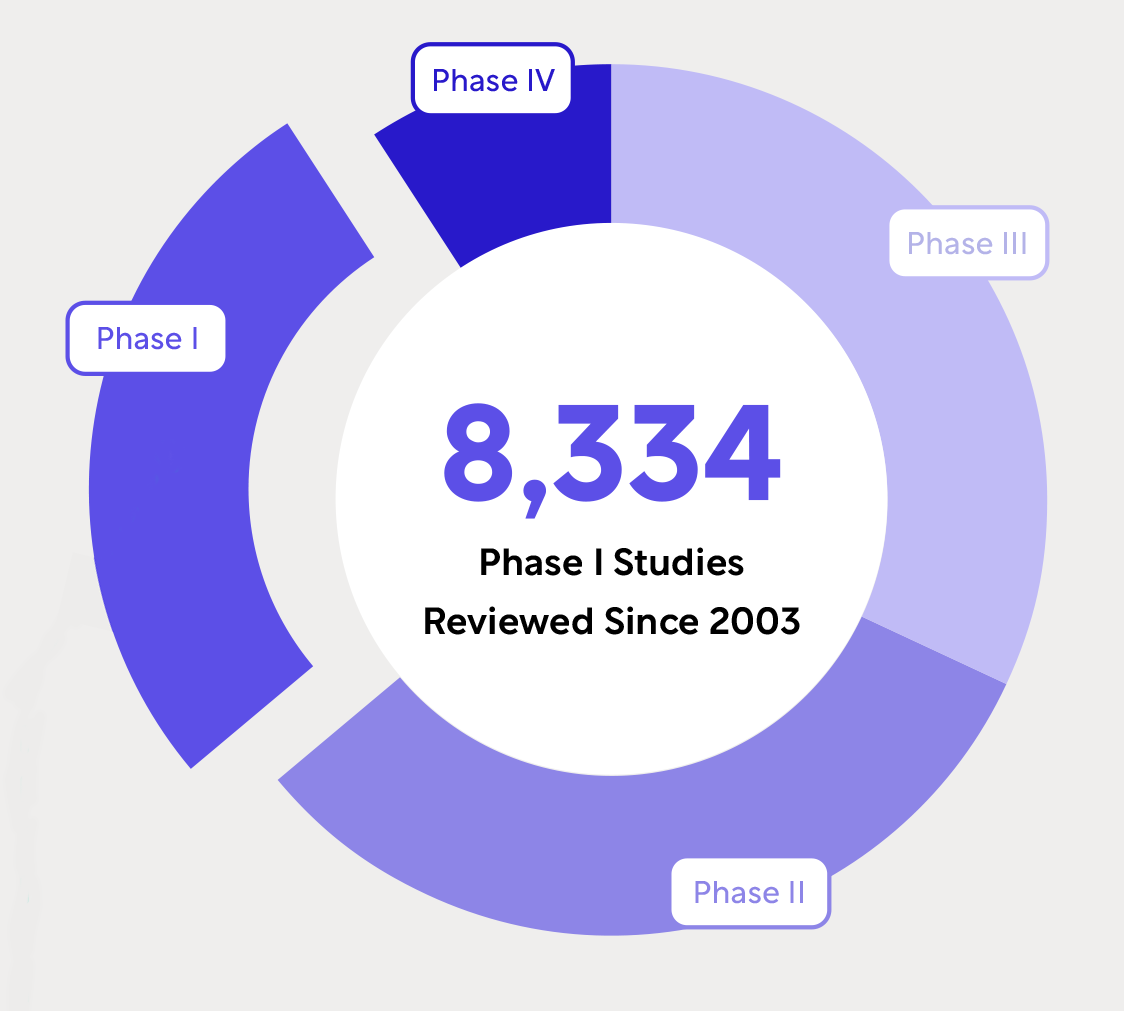
Ethics in Clinical Research
WCG’s Unmatched Experience in Early Phase Hematology and Oncology
Case Studies
Ethics in Clinical Research