Ethics in Clinical Research

Ethics in Clinical Research
Why Was My Research Submission/Protocol Deferred? And What Should I Do?
Blog Posts
Ethics in Clinical Research
If My Study Includes Approved Drugs, When Do the Risks of Those Drugs Need to Be Disclosed in the Consent Form?
Blog Posts
Diversity & Inclusion
FDA’s Path Toward Diversity in Clinical Trials: The DEPICT Act and Sponsor Responsibility
Whitepapers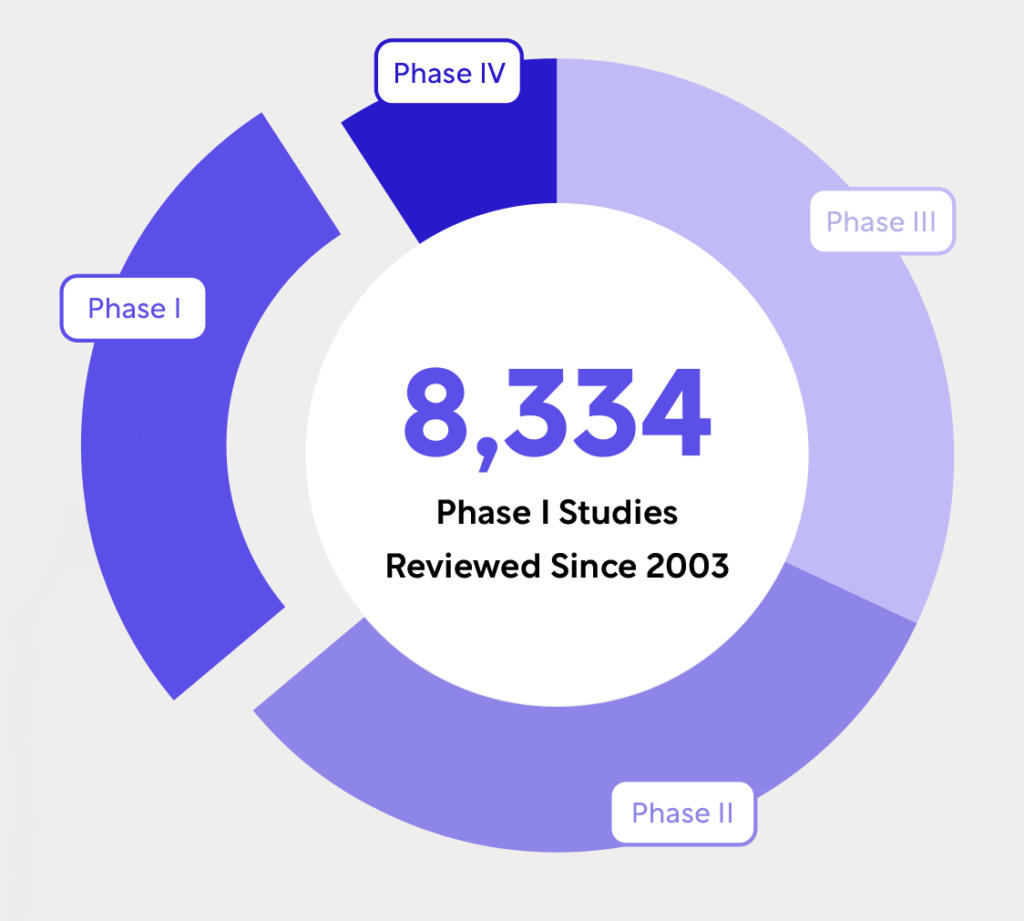
Ethics in Clinical Research
WCG’s Unmatched Experience in Early Phase Hematology and Oncology
Case Studies
Ethics in Clinical Research
Delve into the World of Psychedelic Research and Ethical Inquiry
Podcasts
Ethics in Clinical Research
IRB Submission Requirements for Decentralized Clinical Trials
Blog Posts
Clinical Trial Safety
The Nuances of Patient Selection: Why Some Trials Need Eligibility Adjudication
Whitepapers
Ethics in Clinical Research
Age-based Exclusions in Clinical Trials
Blog Posts
Ethics in Clinical Research
Post Study Activities Requiring IRB Review
Blog Posts
Ethics in Clinical Research