Regulatory

Clinical Endpoints
Comment on the revised FDA Industry Guidance for Early AD Drug Development: Implications for Clinical Outcomes Assessments
Blog Posts
Site Efficiency
WCG’s 2024 Clinical Research Site Challenges Report
Whitepapers
Series: WCG Talks Trials
Amplifying Patient Voices to Advance Clinical Research: The Importance of Patient Advocacy
Podcasts
Diversity & Inclusion
The Importance of Diversity, Equity, Inclusion, and Intersectionality in Clinical Research
Podcasts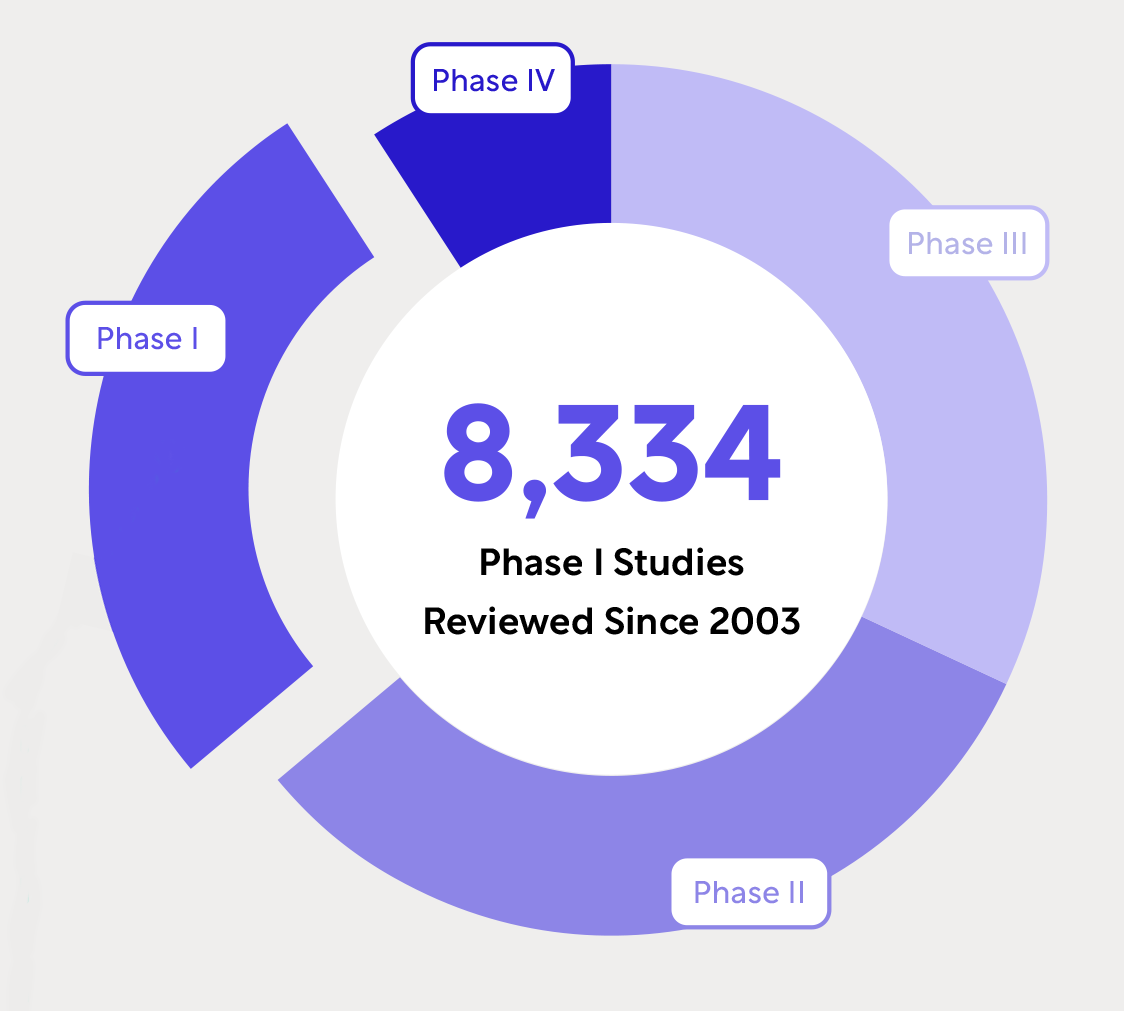
Ethics in Clinical Research
WCG’s Unmatched Experience in Early Phase Hematology and Oncology
Case Studies
Clinical Trial Safety
The Nuances of Patient Selection: Why Some Trials Need Eligibility Adjudication
Whitepapers
Site Efficiency
2023 Clinical Research Site Challenges Survey Report
Whitepapers
FDA & ICH
Function over Form: Assessing Different Consent Form Formats
Whitepapers
Cell & Gene Therapy
Why Biotech Sponsors Need Outside Support: IRB, IBC, DMCs and EACs
Whitepapers
Diversity & Inclusion