Case Studies

Participant Recruitment
WCG Increases Enrollment by 34% for Major Depressive Disorder Study
Case Studies
Participant Recruitment
WCG Increased Enrollment by 67%
Case Studies
Participant Recruitment
99% Enrollment Contribution to Decentralized Trial
Case Studies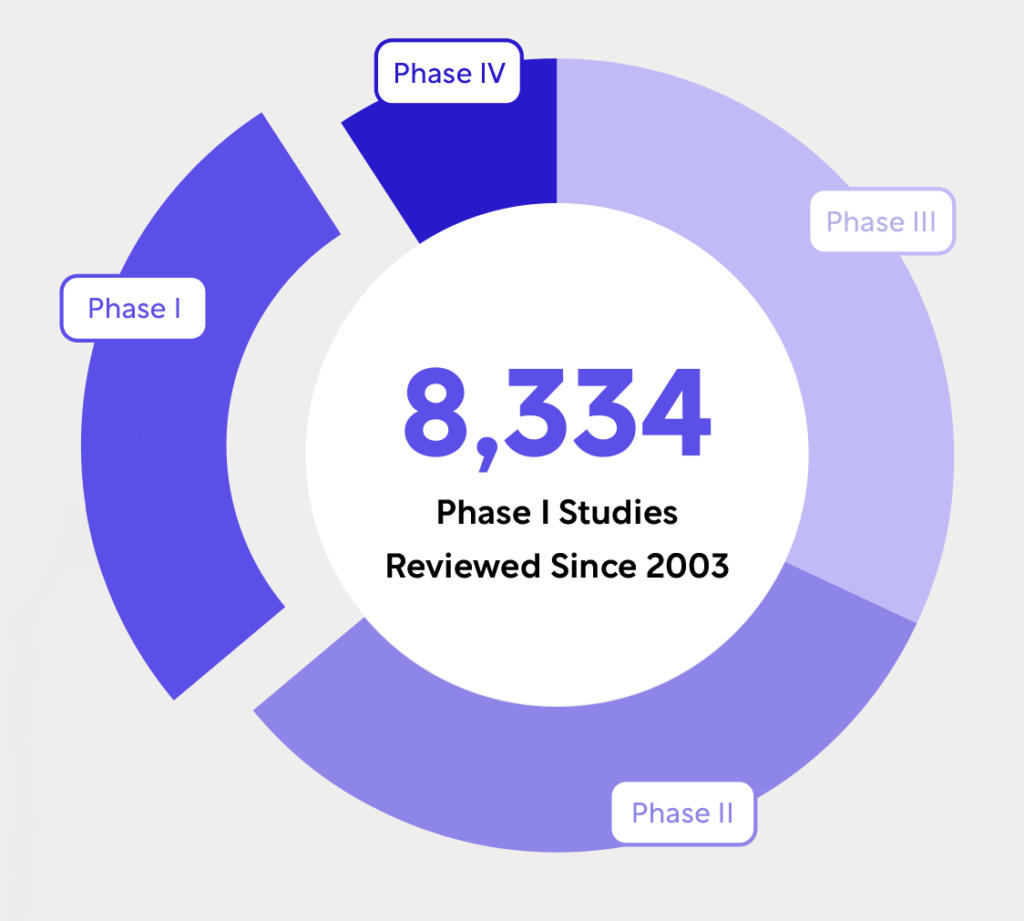
Ethics in Clinical Research
WCG’s Unmatched Experience in Early Phase Hematology and Oncology
Case Studies
WCG Provided Critical Support for Pediatric Rare Disease Trial, Protecting Endpoints, Ensuring Data Validity and Meeting FDA Milestones
Case Studies
WCG Improves PANSS Data Quality and Signal Detection in a Global Schizophrenia Trial, Leading to Approval
Case Studies
A Top 10 Pharma Deploys Just-In-Time Training Across 1,000+ Sites
Case Studies
Site Efficiency
Clinical Trial Training Delivery for Remote Audiences
Case Studies
Participant Recruitment
Mid-sized Biopharma Closes Enrollment 3 Months Ahead of Schedule
Case Studies
Clinical Trial Safety