Blog Posts
Explore timely perspectives from WCG’s clinical trial operations and scientific leaders.

Participant Recruitment
Is IRB review required when linking to clinicaltrials.gov postings on patient advocacy websites and newsletters?
Blog Posts
Clinical Endpoints
Developing Psychedelics for Use in CNS Disorders
Blog Posts
Improve Training, Increase Participation, Reduce Redundancy, & Cut Costs: Why Virtual Site Training is the New Norm
Blog Posts
Ethics in Clinical Research
Do parents need to accompany adolescents at each research-related office visit?
Blog Posts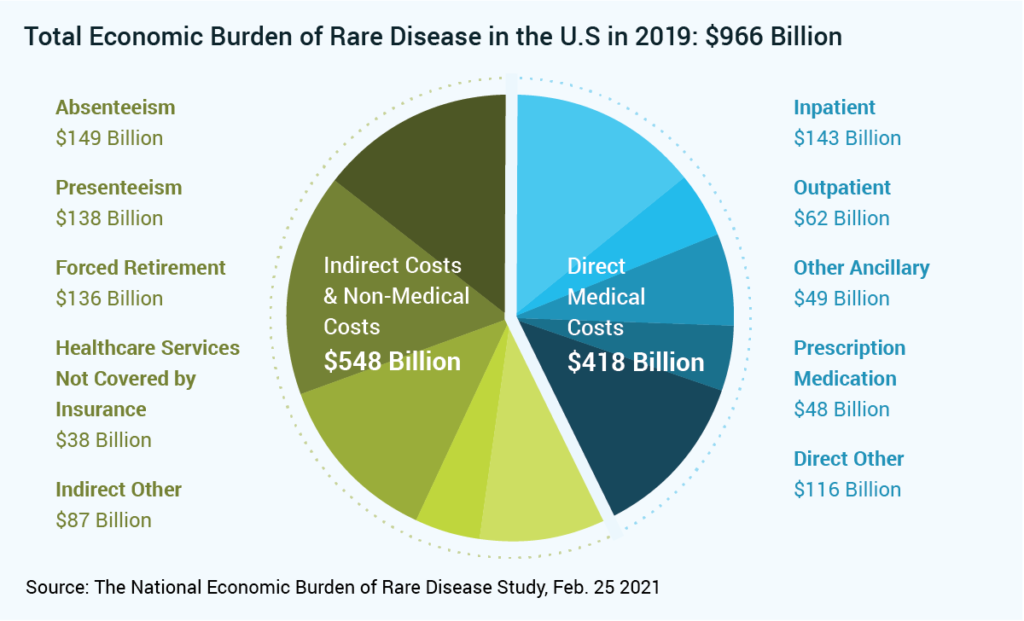
New Study Uncovers the $966 Billion Economic Impact of Rare Diseases
Blog Posts
Cell & Gene Therapy
As a sponsor, does my HGT clinical trial require IBC approval at all sites?
Blog Posts
Cell & Gene Therapy
Does Human Gene Transfer research at my site require IBC approval?
Blog Posts
FDA & ICH
When should a child assent form be used in a pediatric clinical trial?
Blog Posts
Clinical Trial Safety
What are IRB submission requirements for post marketing safety reports?
Blog Posts
Ethics in Clinical Research