Research Sponsors

Ethics in Clinical Research
What Information Must Be Included in the Cost Section of an Informed Consent Form?
Blog Posts
WCG Revolutionizes Ethical Review Process with IRB+ Service, Achieving Unprecedented Efficiency Gains
News
Ethics in Clinical Research
Why Was My Research Submission/Protocol Deferred? And What Should I Do?
Blog Posts
Series: WCG Talks Trials
Amplifying Patient Voices to Advance Clinical Research: The Importance of Patient Advocacy
Podcasts
Ethics in Clinical Research
If My Study Includes Approved Drugs, When Do the Risks of Those Drugs Need to Be Disclosed in the Consent Form?
Blog Posts
Diversity & Inclusion
FDA’s Path Toward Diversity in Clinical Trials: The DEPICT Act and Sponsor Responsibility
Whitepapers
Diversity & Inclusion
The Importance of Diversity, Equity, Inclusion, and Intersectionality in Clinical Research
Podcasts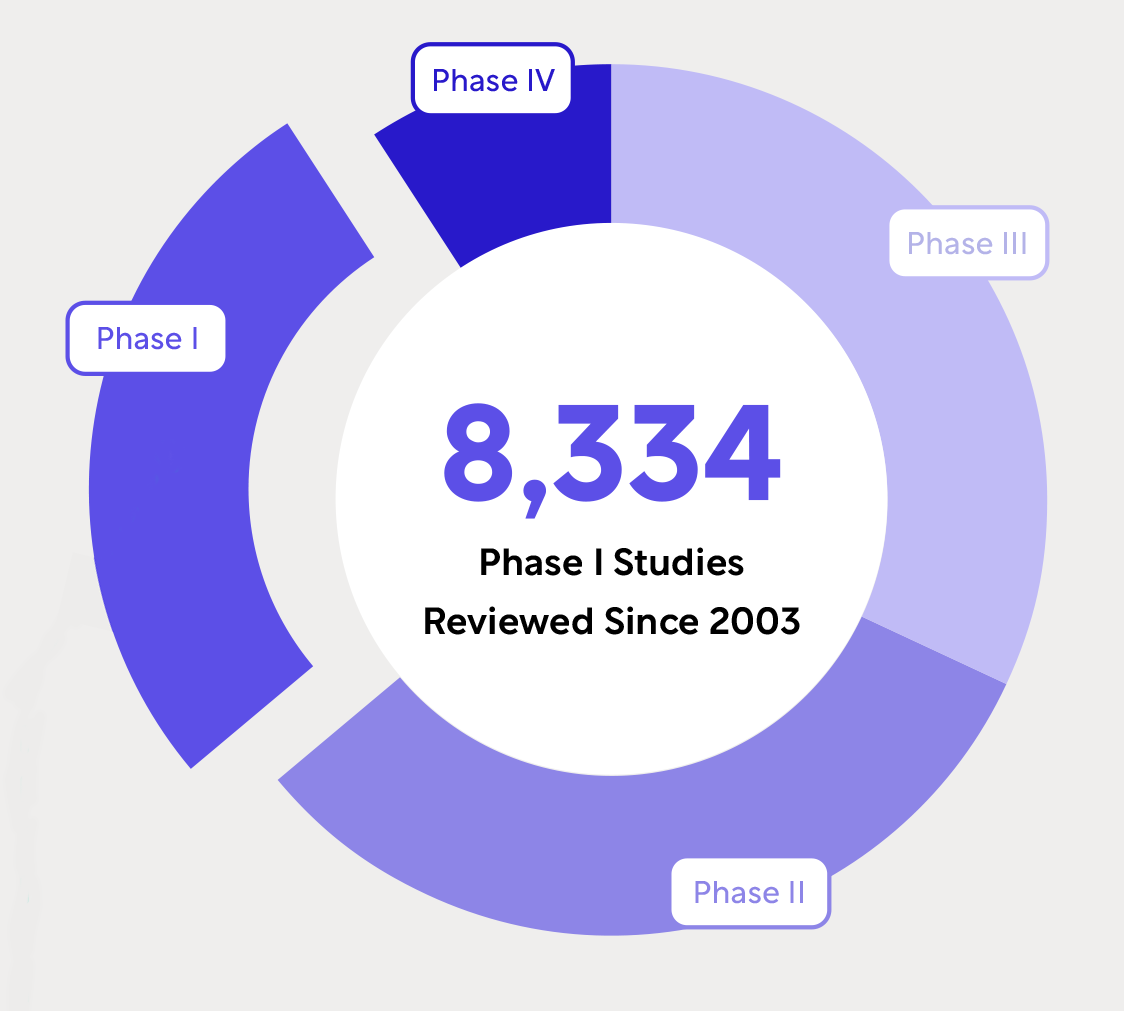
Ethics in Clinical Research
WCG’s Unmatched Experience in Early Phase Hematology and Oncology
Case Studies
Ethics in Clinical Research
Delve into the World of Psychedelic Research and Ethical Inquiry
Podcasts
Clinical Trial Safety