Research Sites & Institutions

What elements of Informed Consent must we include when pre-screening?
Blog Posts
Ethics in Clinical Research
Does the IRB need to review news stories?
Blog Posts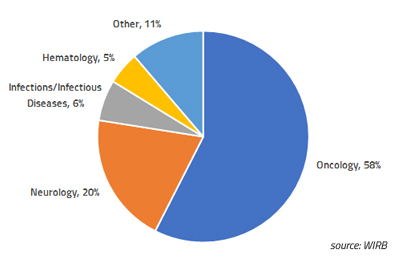
Single-Patient Expanded Access: WIRB experience in 2018
Blog Posts
Limited IRB Review: Are You Prepared for January 21st?
Blog Posts
Site Efficiency
IRBs can make the most of central IRB partnerships
Articles
Clinical Trial Safety
Infectious Disease Challenge Studies: Ethical Issues in Causing Disease for Medical Knowledge
Whitepapers
WIRB-Copernicus Group Acquires KMR Group and Metrics Champion Consortium (MCC)
News
FDA & ICH
Is This an Interventional Clinical Trial or Observational Study? How- and Why- It Is Important to Write Protocols That Make This Distinction Clear
Whitepapers
Ethics in Clinical Research
Clinical Trial Recruitment Practices: The Evolution of Ethical Considerations
Whitepapers
Ethics in Clinical Research