The following Insight is a featured article from WCG’s 2025 Trends & Insights Report. If you would like to read more insights from this report, please click here.
Former U.S. President Obama called precision medicine, “Health care tailored to you.”1 Nowhere is precision medicine more critical than in oncology, where life and death clinical decisions are based on an individual’s genetic or biomarker results. In the last decade, precision medicine approaches made a paradigm shift in the understanding and treatment of cancer.2 From “one-size-fits-all” to an individual, biomarker-driven treatment, precision medicine improved treatment outcomes and patient survival rates, while reducing toxicity.3
Despite tremendous progress, much is left to do. The number of biomarkers is vast and complex. A disease biomarker may be a combination of factors, such as multiple genes and proteins barely detectable. Research study designs have been evolving to meet these needs, with the classic basket and umbrella master trial designs requiring biomarker validating components. Similarly, increased complexity with changing master trial designs may move science forward more efficiently. For example, NCI-MATCH has evolved into ComboMATCH to address the issues of tumors having more than one gene driver and cancers developing resistance to treatment.4
Furthermore, biomarkers and precision medicine approaches will evolve as disease treatments evolve. Next-generation, multi-gene DNA sequencing will grow into multi-RNA sequencing and multi-modal panels, including nucleic acid and other omics-based target testing. Precision medicine requires biomarker-driven targeted treatment, and with cancer’s heterogeneous nature, further profiling of patient tumor tissues will be required.
The standard collection of tissue and blood samples for DNA will commonly add RNA sequencing, gene expression, mutation and deletion profiles, protein expressions, immune repertoires, tumor microenvironment, and metabolic changes to drive cancer treatment’s increased rate of success. The precision medicine approach works hand in glove with drug development. The biomarkers found in tyrosine kinases, EGFR, ALK, KRAS mutations, immune checkpoints, and T-cell targets resulted in the approval of products targeting those biomarkers. That trend will continue and mature.
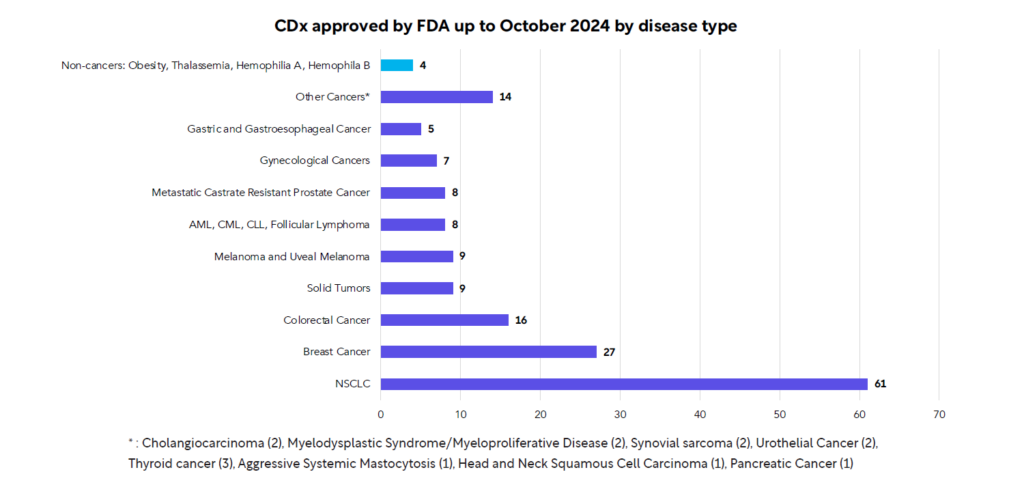
Ensuring biomarkers achieve their potential requires the assays to detect them are high quality, accurate and safe and effective. When used to identify patients who are most likely to benefit, be at increased risk, or need monitoring from a particular product, the assay to detect the biomarker is a companion diagnostic device (CDx). These CDx provide information essential for the safe and effective use of a corresponding drug or biological product. As of October 31, 2024, there are 168 FDA-cleared or approved CDx (In Vitro and Imaging Tools).5 Out of the 168 FDA-cleared or approved CDx, 164 (97.7%) are for oncology indications and only four (2.3%) are for non-oncology indications. (see Figure 1).
The FDA, as always, will be advancing with the technology. This started with the 21st Century Cures Act detailing the Oncology Center of Excellence.6 Bringing together the expertise across drug and device realms for the trials testing companion diagnostics and platform treatment approaches will require exemplary coordination. For example, developing the next decade of gene therapy products will mean testing off-the-shelf CAR-T products with biomarker-directed targets. Institutional Review Boards (IRBs) will also need to keep pace and realize last year’s exploratory objectives are next year’s companion diagnostics, staying current on the technology and regulatory components. Only in this way can we all support the true value of precision medicine.
References:
- The White House. “Precision Medicine.” The White House, https://obamawhitehouse.archives.gov/precision-medicine.
- Rulten, S.L.; Grose, R.P.; Gatz, S.A.; Jones, J.L.; Cameron, A.J.M. The Future of Precision Oncology. Int. J. Mol. Sci. 2023, 24, 12613. https://doi.org/10.3390/ijms241612613
- Nature. “Milestones in Cancer.” https://www.nature.com/immersive/d42859-020-00083-8/index.html
- 4National Cancer Institute. “New NCI Precision Medicine Trials.” Cancer Currents Blog, https://www.cancer.gov/news-events/cancer-currents-blog/2023/new-nci-precision-medicine-trials.
- U.S. Food and Drug Administration. “List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools).” https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools.
- U.S. Food and Drug Administration. “Oncology Center of Excellence.” https://www.fda.gov/about-fda/fda-organization/oncology-center-excellence.
Related Insight:

Under the Microscope: Biomarker and Diagnostic Tests as FDA-Regulated Devices
WhitepapersLearn more about our Trends and Insights Report for 2025
Fill out this form to contact WCG with any questions and comments, to learn more about our authors and insights into the 2025 Trends and Insights Report.
