Clinical Research Trends & Insights for 2023
In our 2023 preview, 14 experts from WCG share the important shifts, trends, regulations, and priorities that will inform clinical trial development this year and beyond.
After the disruptions to the clinical research field in 2020 and 2021, many looked forward to 2022 as a year of “getting back to normal.” Instead, we found that many pandemic-associated changes have forced us to look at a lot of issues through a new lens.
Certainly, “decentralized trials” dominated our conversations, and considerations of the importance of proactive efforts to facilitate the inclusion of historically underrepresented populations in research is finally being recognized. Both biopharma sponsors and clinical sites are finding their footing again, allowing them to renegotiate some old practices from new perspectives (e.g., investigator oversight responsibilities in decentralized trials, how much of site monitoring really needs to be on-site).
Our clinical trials continued to evolve in response to new therapeutic advances, with more protocols incorporating complex design elements, earlier evaluations of efficacy, and an increasing number of platform studies to move potential drug candidates to decisions more quickly.
2023 looks like it will be just as exciting. In this report, WCG experts from a variety of areas talk about what they’re watching, expecting, and looking forward to in 2023. We’re exploring study design, study conduct, new proposed regulations and guidance, and even at the types of therapeutic agents moving into clinical investigation.
Thank you for joining us as we look ahead to the next exciting year!
Explore Insights:
Perspectives on Clinical Research Sites in 2023
Redesigning the Clinical Research Landscape
Diversity Strategies and Empowering Sites to Support Them
The FDA’s Recent Notice of Proposed Rulemaking (NPRM) and Guidance
Diversifying Clinical Research by Providing Research Participants Payments
Clinical Trial Technology Integration Now and Into the Future
Interoperability Required for the Future of Clinical Trials
Quality by Design and Slowing Down to Speed Up
The Emerging Use of Adjudication in Oncology for Determining Patient Eligibility
Current Trends in Neurodevelopmental and Rare Pediatric Disease
Neuroscience Research Trends to Pay Attention to in 2023
Epilepsy Treatments and Research: Where we Started and Where we are Heading
Watch our 2023 Trends & Insights Webinar
Hear our online panel of clinical trial experts discuss the future of clinical trials, and answer questions from your peers.
This engaging discussion is facilitated by Jill Johnston, Chief Innovation Officer of WCG.

Perspectives on Clinical Research Sites in 2023

Sandra Smith, RN, MSN, AOCN
Senior Vice President, Clinical Solutions & Strategic Partnerships,
WCG
To combat the tumultuous conditions of the industry, research organizations have been forced to look internally to identify operational efficiencies over the last two years, and institutional reconfiguration will continue to evolve in 2023. Centralization of research services, embedding enabling technologies, and establishing research partnerships – both with service providers and with sponsors – will remain key themes in addressing site capacity challenges. Many non-institutional research sites will aggregate into networks to achieve higher operational performance.
Human capital remains critical, and the skillset for research expertise will continue to be in high demand in 2023. Sites are reporting fewer vacant positions, but turnover will persist, and training the newly hired, less experienced research personnel will remain critical. Sponsor-supported staffing augmentation solutions will be key to trial conduct for many organizations.
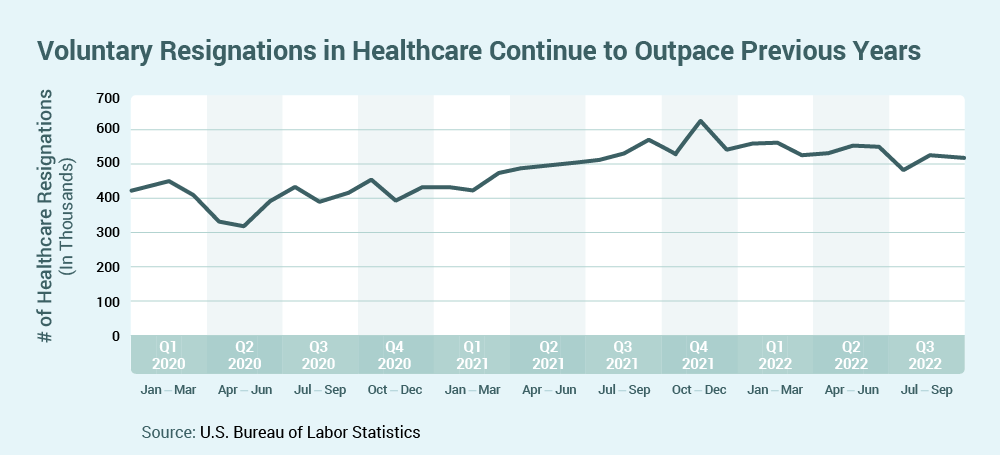
Oncology will endure as a dominant therapeutic area in clinical research in 2023. Approximately 50 percent of all clinical trials started in 20221 were in oncology, with more than 1M patients (about the population of Delaware) needed to complete those open trials2. Increasing trial complexity will challenge sites with narrow inclusion/exclusion criteria, multiple study arms (i.e., 19-20 percent increase in four to five arm trials in oncology)3, and an increasing number of amendments per clinical trial (i.e., 57 percent increase since 2017)4.
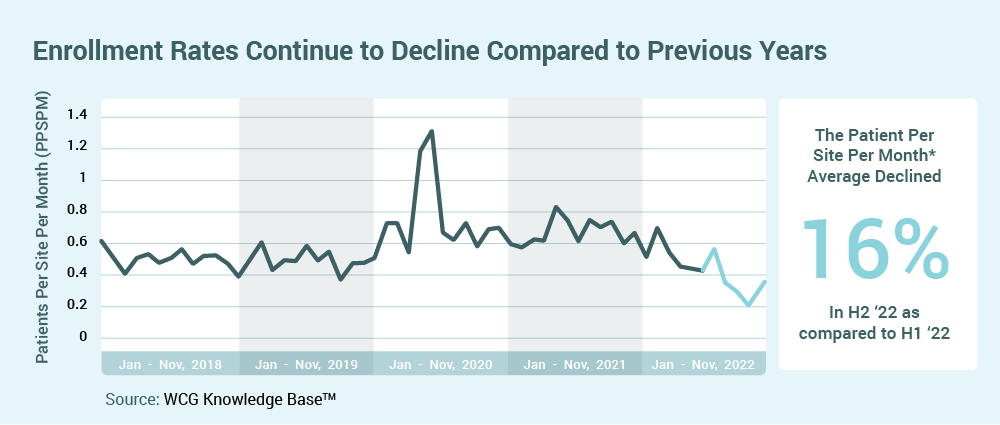
Declining average rates of enrollment per site5 and the need for increased enrollment diversity will accelerate sponsor interest in opening trials at community-based sites and in supporting new investigators/sites. Rapid trial activation, bringing trial access to new participant populations, and utilization of decentralized trial components, will be critical components for trial success.
References:
1, 2, 3, 4, 5 WCG Knowledge BaseTM, 2022
Redesigning the Clinical Research Landscape

Jamie Harper, MHA
Director of Site Engagement and Relations, WCG
In 2023, clinical trial access and improving participant diversity will remain key concerns within the clinical research and healthcare community. Compounded by the number of physician investigators continuing to decrease, the industry will be tasked with developing solutions to ensure scientific medical advancements continue while supporting these diverse populations. Identifying and supporting new physician investigators at research sites, especially those ingrained into the community setting, is critical to offsetting these concerns and accelerating advancements.
Redesigning the clinical trial landscape to mitigate these challenges and create an environment that allows engaged community physicians to provide clinical trial access to the communities they serve is a must. By engaging community sites and physicians who may not have previously considered clinical research as an option, it presents a path toward addressing the concerns of clinical trial access and participant diversity. However, this pathway will require the clinical research industry to provide support for the tools and mentorship needed to successfully conduct compliant clinical trials.
The new environment will require evolution of the current collaborations between sponsors, clinical research organizations (CROs), third-party clinical research vendors, and experienced physician investigators. This collaboration’s success will depend on a shared focus to cultivating a redesigned clinical research landscape through inclusion.
Diversity Strategies and Empowering Sites to Support Them

Katherine Cornish, PhD, PMP
Former Associate Director, Patient Advocacy & Clinical Trial Diversity,
WCG
As the FDA (U.S. Food and Drug Administration) moves closer to finalizing the current draft guidance on establishing diversity plans1 to improve clinical trial participation of underrepresented racial and ethnic populations, it becomes imperative to ensure that the strategies outlined in these plans are translated to the site level in intentional and actionable ways.
Sponsors must empower their sites by providing pragmatic strategies that site staff can use to effectively identify, engage, enroll, and retain underrepresented populations. This should be done through the creation of site-focused diversity plans that tie into the overarching diversity plan. Key partnerships with advocacy groups, community-based organizations, and other similar entities outlined in diversity plans at a national level should be translated to individual sites locally. Sponsors must also provide the necessary support to ensure these strategies can be enacted by their sites.
Additionally, these strategies must be identified, documented, and mobilized early in the trial lifecycle; they must be thought about proactively rather than as rescue methods. Sponsors should reach out to the FDA to discuss their diversity plans as early as possible – certainly prior to study start. Thinking about these approaches early on can also ensure that the necessary support is preemptively budgeted at the site level, allowing sites to initiate local advocacy and community engagement efforts from the start. This ensures that sites are prepared and ready when recruitment begins and can enact their action plans from first patient to last visit.
References:
- https://www.fda.gov/media/157635/download
The FDA’s Recent Notice of Proposed Rulemaking (NPRM) and Guidance

Dave Borasky, MPH, CIP
Vice President, IRB Compliance
WCG IRB
The year 2022 ended with two interesting developments for Institutional Review Boards (IRBs) and industry on the FDA front. First, the FDA published two Notices of Proposed Rulemaking (NPRMs)1,2 that are intended to bring greater harmony between the FDA regulations for human subjects research, and the Common Rule regulations that apply to U.S. federally funded research that is not subject to FDA regulation. While this harmonization is expected – it was mandated by the 21st Century Cures Act – it was not clear when the NPRMs would be issued. Harmonization on issues of informed consent requirements and the use of a single IRB for multisite trials will improve the review and implementation of clinical research in 2023 and beyond.
The second late-year offering from the FDA was the draft guidance Ethical Considerations for Clinical Investigations of Medical Products Involving Children. Through this guidance, the FDA has finally provided clear direction on the expectation that IRBs use component analysis when reviewing research with children that includes multiple research-related interventions or procedures. Component analysis, which has the IRB assess each study procedure (and each study arm), considering the risks and benefits, is often confusing to sponsors. The guidance also provides direction on the FDA’s thinking on what constitutes a “minor increase over minimal risk” (21 CFR 56.102(i)), which is not defined in the regulation, but represents an important threshold for reviewing research involving children, particularly in the context of component analysis.
References:
- https://www.federalregister.gov/documents/2022/09/28/2022-21088/protection-of-human-subjects-and-institutional-review-boards
- https://www.federalregister.gov/documents/2022/09/28/2022-21089/institutional-review-boards-cooperative-research
Diversifying Clinical Research by Providing Research Participants Payments

Kelly Fitzgerald, PhD
IRB Executive Chair and Vice President, IBC Affairs
WCG IRB
Payment to research participants is a critical aspect of diversifying clinical research participation. There will be a continued increase in awareness of this issue in 2023. IRBs are often seen as a barrier to paying participants, and that may have been true in the past, but the thinking on this topic has evolved significantly in the last few years, informed in part by the recognition of the importance of research participants as partners rather than as research “subjects.”1
IRBs are tasked with ensuring that research-recruiting and consenting processes do not exert undue influence on participants, and high payments may be seen by IRB members as unduly influential2. Some people believe that payments could incentivize someone to participate in activities they would otherwise choose not to do, particularly people with low incomes. However, when an IRB insists on lower payments, participants who have less free time, less available income, or more burdensome lives are less likely to participate, and the study ends up with a participant population that does not match society because those people who can bear the financial burdens of research participation will participate.
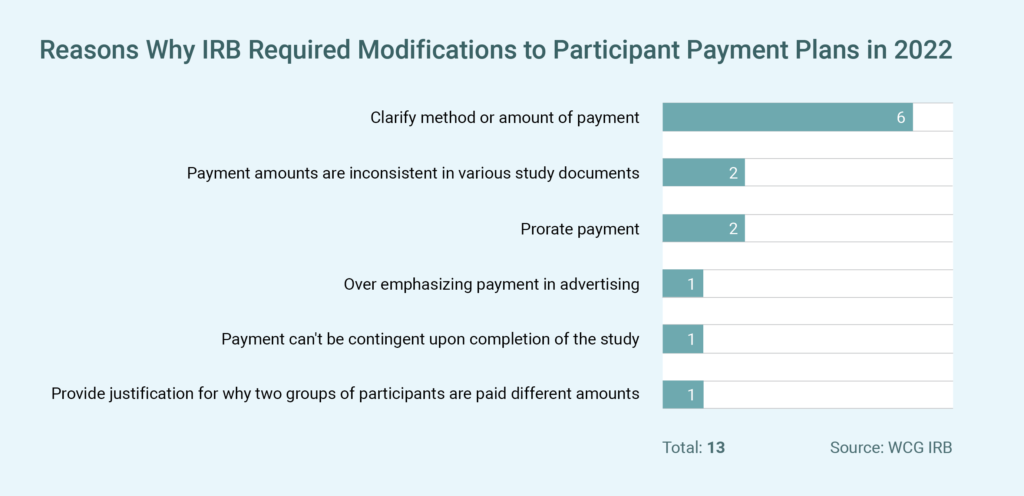
The clinical research world now recognizes that payments can incentivize without being unduly influential. While there is a perception that IRBs will not approve high payments for participants, this is changing. During the last 24 months, out of approximately 10,000 IRB reviews, only 13 reviews resulted in the IRB specifically requiring modifications to the payment plan (see table), and no records included a request to decrease the proposed payments to participants.
IRB members reflect the values and norms of their communities, and the events of the last few years have led to the awareness of the importance, both scientifically and ethically, of diverse representation in clinical trials. To improve in this area, there must be support for higher payments for research participation and a reliance on mechanisms other than limiting payment to ensure participants are recruited ethically. In 2023, there needs to be more discussions around accurately assessing participant costs for research participation and providing just compensation for their service3.
References:
- National Academies of Sciences, Engineering, and Medicine 2022. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Washington, DC: The National Academies Press. https://doi.org/10.17226/26479.
- Payment and Reimbursement to Research Subjects, FDA, Jan 2018.
- Largent EA, Lynch HF. Paying Research Participants: The Outsized Influence of “Undue Influence”. IRB. 2017 Jul-Aug;39(4):1-9. PMID: 29038611; PMCID: PMC5640154.
Clinical Trial Technology Integration Now and Into the Future

Sonia Abrol
Senior Vice President, Product and Strategy,
WCG Velos
The COVID-19 pandemic made many stakeholders in clinical research reassess their technology infrastructure. To sustain and accelerate research, they prioritized taking various research management workflows online to vendor managed systems, ensuring centralized, easy, and secure access to data. As sites and sponsors expanded their technology portfolio, sites are now carrying a bigger technology burden. They are supporting the costs of multiple site technologies and then putting additional time and effort into entering data in both site and sponsor systems. Approximately 90 percent of data needed by sponsors now lives in one or more site systems1.
Additionally, the uptick in adoption of innovative technologies for helping with decentralized trials, patient recruitment, patient retention, and engagement has produced new data silos for sites and sponsors. As a result, clinical research stakeholders are recognizing the need for more interoperability and workflow-driven integrations.
In 2023, sites and sponsors will continue to make technology integration one of their top priorities as they select the right solutions, enhance their technology infrastructure, and budget for such investments. Sites starting their journeys toward integrated ecosystems may begin with key multi-level integrations between their e-regulatory/e-Binder, EHR/EMR, CTMS, IRB and Financial systems. Those that are already ahead of the curve will continue to expand and develop new integration workflows by connecting one silo at a time.
In parallel, technology vendors will continue to work toward offering more open systems and actively defining their own integration roadmaps to help sites and sponsors gain operational efficiencies and reduce the technology burden on users.
Also, it will be interesting to see what the gained momentum in collaboration among sites, sponsors, and vendors will bring in 2023. As these consortia and partnerships come together to help define data exchange standards, map best workflows among site systems and site-sponsor systems and prioritize implementation of cost-effective and standard technology integrations, we will see remarkable growth and acceleration in conducting clinical research and drug development.
References:
- WCG Knowledge BaseTM, 2022
Interoperability Required for the Future of Clinical Trials

Rahul Bafna
Former Chief Product Officer, WCG
The use of healthcare technology at the consumer level has exploded over the past decade. This started with the broad availability and adoption of wearable devices, taking advantage of smaller sensors, better batteries, and always-on connectivity. Over this same time period, there has been a ton of investment in healthcare technology for clinicians – the HITECH Act of 2009 was designed to accelerate medical product development. Many of these advancements in technology have the potential to be transformative in the realm of clinical trials, which for decades have suffered from low patient participation, unrepresentative study populations, heavy operational burden, and long timelines. The key to making these advancements work in real-world settings is the seamless interoperability of patient data.
As an example, patient-trial matching is one of the most time-consuming operations of running a trial. Multiple software platforms exist that can match eligible patients – based on the clinical record stored in one or more EHRs and lab systems – to the various inclusion and exclusion criteria of trials. But for this matching to happen, these solutions need access to those clinical records in a standardized format. Once found, matches need to be pushed back into the physician’s daily workflow – often the EHR – to drive patient conversations, consent and enrollment. Once enrolled, technology can simplify or even automate the data collection necessary for some types of studies. This will be critical for enabling decentralized trials (DCT).
DCTs will allow more patients to participate, potentially from underserved communities who would benefit from trial designs that require fewer or no site visits. The use of technology can also enable more frequent collection of study data, and any data collected in novel systems will need to be pushed back to a unified source, such as an EHR or EDC.
Making sense of clinical data from multiple sources – whether for clinical trials or routine care – requires that data to be collected, retained, transformed, transmitted, standardized, and made available to researchers and clinicians at the right time and in the correct format. Interoperability between all these systems is critical to driving innovation and bringing new treatments to patients. For several years now, the Office of the National Coordinator for Health Information Technology (ONC) has been leading the charge to improve the interoperability of patient clinical data through regulation – mandating specific data standards that enable data to be exported in standardized formats. The 21st Century Cures Act, which includes interoperability standards not only for accessing clinical data but also for enabling new workflows and within platforms like EHRs, will continue to drive advancement in 2023.
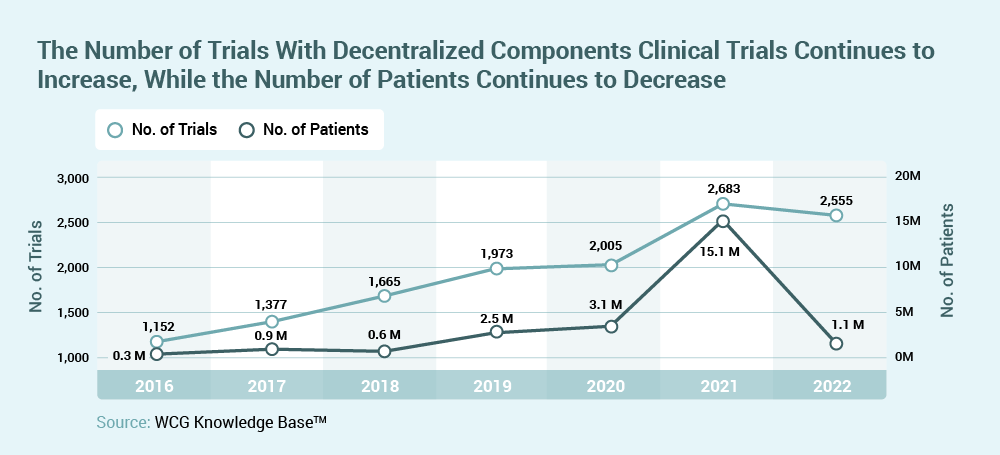
Quality by Design and Slowing Down to Speed Up

Cristin MacDonald, PhD
VP Client Delivery,
WCG Avoca
The year 2023 should be seen as an opportunity for everyone in the clinical research industry to embrace the concept of “slowing down to speed up.” While the concept of Quality by Design is not new, the newly finalized ICH E8 guidance, EU CTR, along with the upcoming ICH E6 R3 and FDA’s Diversity guidance are all examples of the regulators prioritizing efficiency in clinical trial execution by forcing quality into the forefront of study design.
Whether it is by requiring that sponsors show their diversity strategies in advance of launching a pivotal trial, or by subjecting sponsors to regulatory holds for failed submissions, it is time to spend more resources early in study planning. It is time to slow down, think ahead, and ensure quality is built in study design.
Gone are the days of rushing to a first subject in timeframe and reactive protocol amendments that have negative time and budget impacts. Entirely new benchmarks need to be established that encourage study teams to invest the appropriate time and effort in study planning and reward those who demonstrate rapid study completion with minimal amendments by leveraging the proper resourcing up front.
The Emerging Use of Adjudication in Oncology for Determining Patient Eligibility

Shaena Kauffman
Executive Director, Operations EAC, WCG
One of the most interesting trends over the past year has been the increased use of Endpoint Adjudication Committees (EACs) to determine patient eligibility, especially in oncology clinical trials. This trend will likely continue in 2023. Adjudication of inclusion criteria, or eligibility adjudication, has been used in clinical trials where inclusion criteria are subjective. The independent assessment by an EAC gives regulators and scientists confidence in the comparability of clinical trial subjects. Previously, this type of adjudication has been applied to areas such as progressive neurological disease or heart failure, where medical expertise is required, or eligibility is subjective. However, there is a rising trend in using EACs to confirm oncology disease progression to demonstrate a consistent evaluation for patient eligibility and provide real-time feedback to the Investigator study sites.
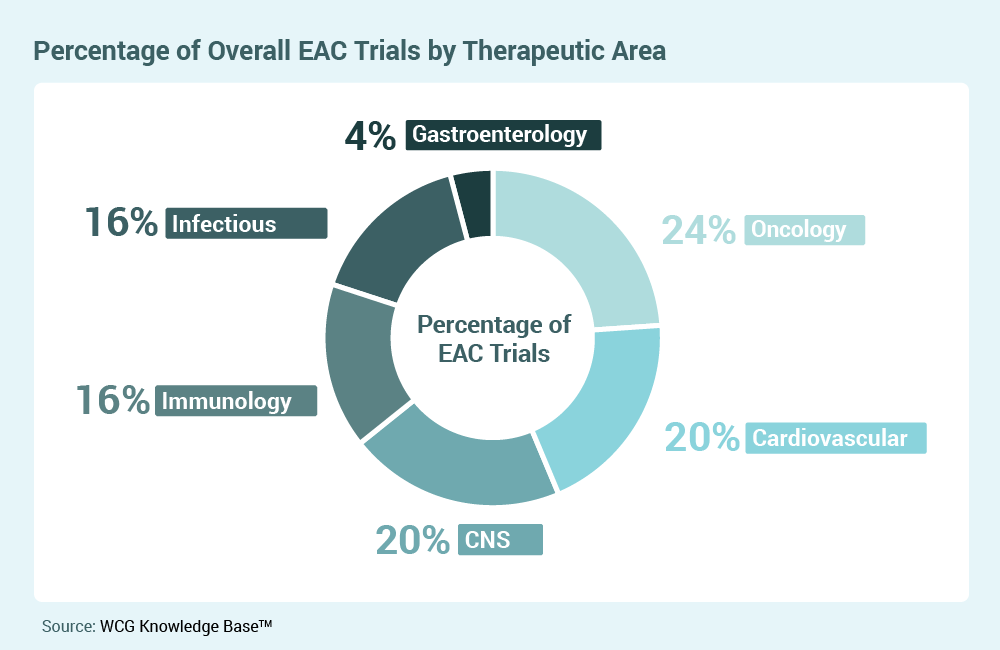
In the area of disease progression, adjudication has the potential to mitigate variability and increase the strength of the clinical trial results. In addition to bolstering the clinical trial data against the regulatory and scientific rigors, EAC members can serve as a resource to investigator study sites that may need access to experts to assess some of the more complex patient eligibility criteria. Of course, the most significant benefits are that adjudication of inclusion criteria reduces protocol deviations and enforces scientific rigor through a pre-randomization, standardized, objective assessment. The use of eligibility EACs will continue to expand in 2023 as sponsors and sites report the benefits.
mRNA: Not Just for Vaccines

Daniel Kavanagh, PhD
Senior Scientific Advisor, Gene Therapy, WCG
Reflecting a new emphasis on cell and gene therapy (CGT) for 2023, the FDA recently announced a new “Super Office,” the Office of Therapeutic Products (OTP), to replace the former Office of Tissues and Advanced Therapies. Included in the announcement are plans to increase hiring and issue more CGT guidance documents in the coming year.
The year 2022 saw many new advances in cell and gene therapy, including FDA approvals for new products and supplemental indications for genetically modified cellular therapies and vectored gene therapies. In 2023, one area to watch closely is that of “vectorless” mRNA therapeutics. Synthetic mRNA is a powerful tool that can program cells for new therapeutic functions. When combined with rapidly developing nanoparticle technologies, mRNA therapeutics may be targeted to a wide array of cell types and tissues. One potential application of gene transfer technology is to bypass the complex in-vitro manufacturing process currently required for biologics and turn the body’s own cells into temporary factories to produce antibodies, enzymes, or vaccine antigens. Many such approaches in development rely on mRNA.
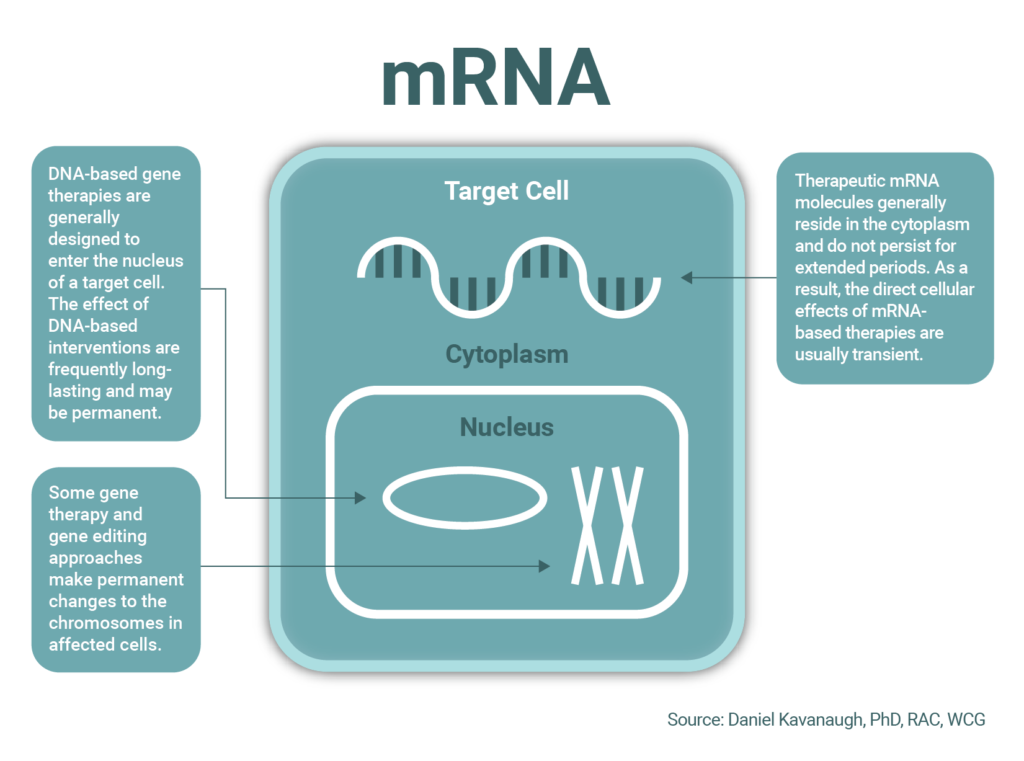
Currently approved gene therapies tend to produce long-lasting or permanent changes in cellular programming and may induce immune responses that prevent re-dosing. For these reasons, many gene therapies are designed for “one-and-done” curative administration. This offers new hope to patients and their families, but also presents challenges for safety, commercialization, and reimbursement. In contrast, mRNA molecules are normally short-lived and exert transient effects on cell biology. Thus, for some applications, mRNA is an important alternative to DNA-based therapies, with potential advantages for safety, re-dosing, and commercialization.
Current Trends in Neuro-developmental and Rare Pediatric Disease

Scott Hunter, PhD
Senior Scientific Expert, Neurodevelopment Disorders,
WCG
In 2023, there will continue to be an emphasis on developing new outcome measures that can be used across rare pediatric and Central Nervous System (CNS) diseases. This has been an area of shared concern among caregivers regarding challenges observed with their impacted children that affect both individual and family engagement and quality of life. Moving from broader cognitive measures that are often experienced as less sensitive, focus has moved more to communication (specifically receptive and expressive language skills in areas including Fragile-X, Angelman, Rett, Autism Spectrum Disorders) and motor control (e.g., Rett, Angelman, Fragile-X).
Seeking greater sensitivity with scales developed, foundation and patient advocacy partners are developing mechanisms to build disorder-specific scales to address key areas of caregiver and patient concern, while also collaborating across indications to support better measurement design. The goal of this is to amplify acceptance by the FDA of caregiver and clinician report measures (ClinROs and patient reported outcomes, or PROs) in the rare disease space.
We will move toward using wearables and engaging more directly with samples collected from these technologies that can be expert reviewed and rated. Collecting simultaneous biological and behavioral outcomes through direct movement assessment, recording of communication efforts, and video monitoring of responses, provides significant multipoint data that can be aggregated in tandem with use of performance measurements in rare and neurodevelopmental disease.
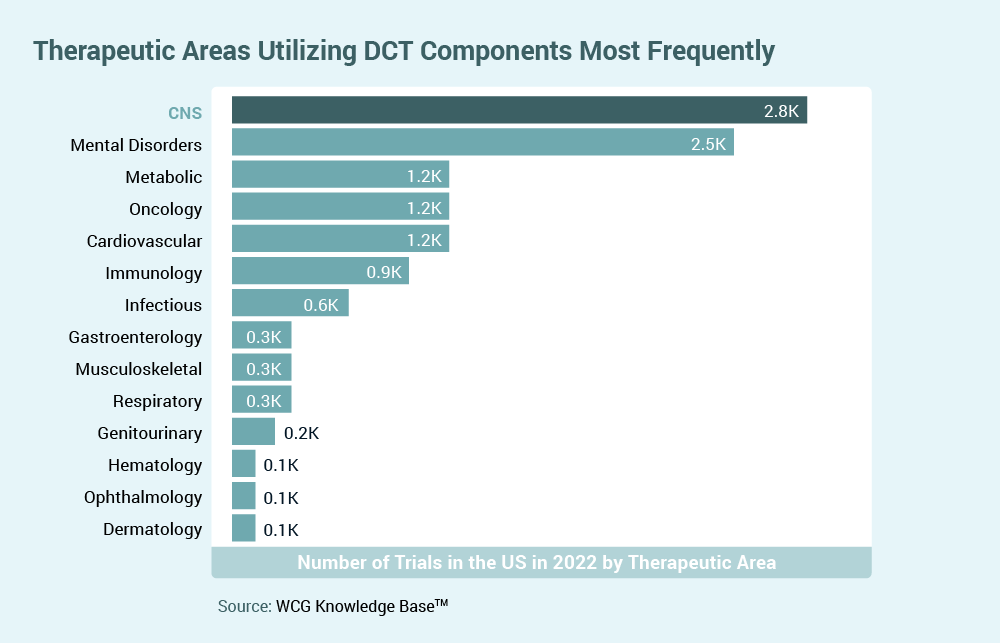
Neuroscience Research Trends to Pay Attention to in 2023

Mark G. A. Opler, PhD, MPH
Chief Research Officer, WCG Endpoint Solutions
The last five years have brought a remarkable burst of innovation in neuroscience drug development. From the approval of the first, rapid-acting agent to treat depression, to the past year’s massive surge of activity in the study of psychedelic compounds, to the ongoing, exploratory use of wearables and other technology-oriented methods for data collection, there has been a tremendous degree of change. In the coming year, we’ll see some of these exciting yet unproven innovations begin to demonstrate their promise in a more substantive way with enduring, real-world impacts.
A few of the most important trends to watch include:
- Late-Stage Studies of Psychedelics and Cannabinoids: The first large-scale, Phase III industry-sponsored studies of psilocybin by Compass Pathways should help prove the viability of the psychedelics movement in neuroscience. The published results of the Compass Phase II program, if confirmed in Phase III, would solidify this while also clarifying the direction that this exciting new class of treatments is likely to take in years to come.
- Development of Wearables and “Digital Biomarkers”: Although regulatory agencies have held off on explicitly approving the use of novel endpoints related to wearable devices or similar device-rated digital biomarkers, the proliferation of these tools as exploratory endpoints is creating a growing wealth of data. As the evidence base for them continues to grow, standardization and adoption will almost certainly follow.
Epilepsy Treatments and Research: Where we Started and Where we are Heading

Mike Cioffi
Senior Vice President, Clinical Solutions and Strategic Partnerships,
WCG
Currently, there are more than 30 antiseizure medications (ASMs) available to clinicians to treat patients. But even with such a significant amount of treatment options, there are still over 30 percent of individuals who do not respond to common ASMs and are addressed as “drug-resistant.”1 This will drive innovation in both pharmacological and non-pharmacological interventions aimed at improving symptoms and quality of life for patients along with their caregivers.
Research is ongoing in many areas, and we will see important advances in the field in the coming years. An increased application of technologies to aid in epilepsy management is likely. Smartwatches that can detect imminent seizures and alert caregivers to the location of the patient, and apps that can inform clinicians with real-time information on their patients’ status and seizure counts are all on the horizon.
Precision medicine has begun to take a more prominent role as we understand more about the pathogenesis of epilepsies. Significant advancement has been made in identifying the genetics of epilepsies, and the discovery of gene mutations responsible for a large portion of patients with developmental and epileptic encephalopathies (DEEs)2.
Additionally, the search for biomarkers to guide drug development is moving at a fast pace and will potentially allow clinicians to improve diagnostic accuracy, predict response to ASMs, and improve outcomes for patients.
While there are certainly limitations to current technologies and biomarker identification, and despite precision medicine being further researched, this multidisciplinary approach will redefine our ability to improve the treatment and management of epilepsy in the future.
References:
- Kalilani L, Sun X, Pelgrims B, Noack-Rink M, Villanueva V. The epidemiology of drug-resistant epilepsy: a systematic review and meta-analysis. Epilepsia. (2018) 59:2179–93. doi: 10.1111/epi.14596
- Perucca P, Bahlo M, Berkovic SF. The genetics of epilepsy. Annu Rev Genomics Hum Genet. 2020;21(1):205–30
Request a Meeting with a WCG Expert
Interested to learn how the insights above will affect your studies? Submit this form to request a meeting with one of WCG’s consultants featured in this report.