WCG’s IBC+ Optimizes Biosafety Processes for Faster Study Starts
Comprehensive Oversight. Expert Support. Streamlined Workflows.
Don’t delay your gene transfer clinical trial with traditional Institutional Biosafety Committee (IBC) models that can be inefficient. Clinical trials involving gene transfer, including cell and gene therapy, chimeric antigen receptor T-cell (CAR T), genome editing, clustered regularly interspaced short palindromic repeats (CRISPR), and messenger ribonucleic acid (mRNA), continue to grow, and most of these trials require approval by an IBC.
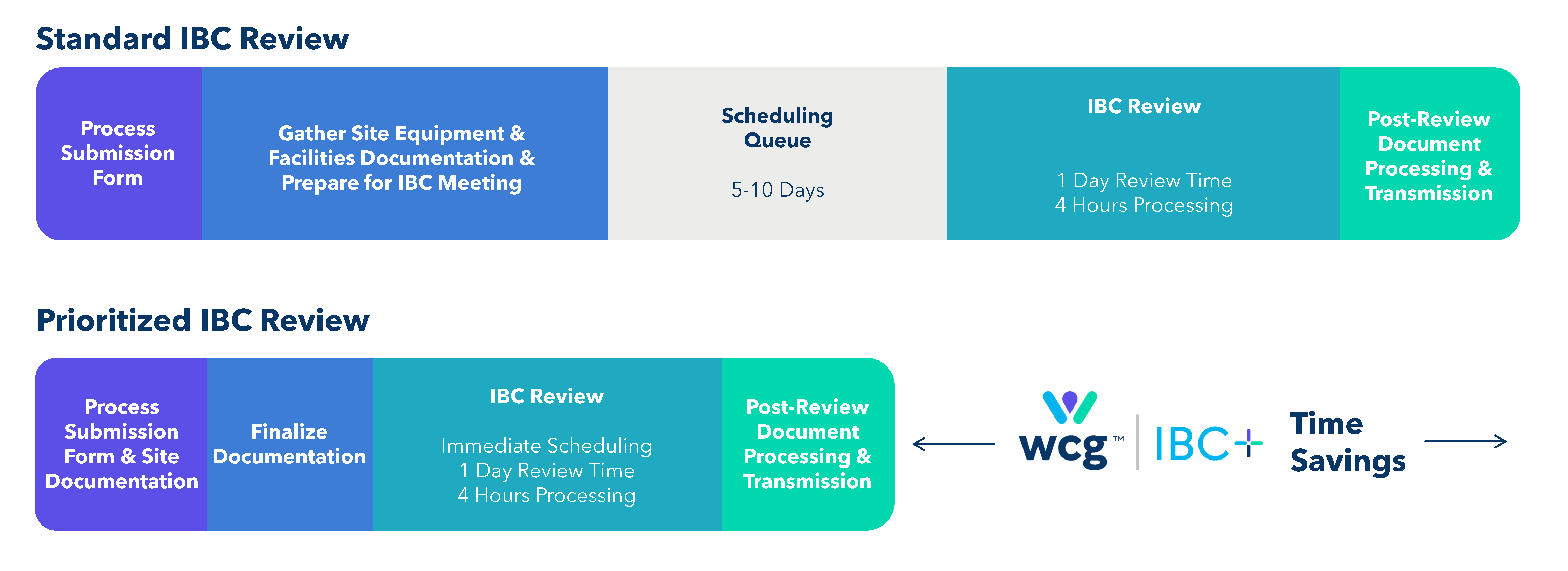
Now, with WCG’s IBC+, you can ensure faster and more efficient IBC reviews, without compromising the rigor of biosafety oversight. WCG’s IBC+ is reinventing standard document processing, which has resulted in up to 50% faster turnaround times and shortened site activation by multiple months.
Reinventing Biosafety Oversight to Advance Your Gene Transfer Studies
Accelerated Timelines
Realize the benefits of reducing turnaround times by up to 50%. With streamlined IBC processes, you can start your study faster and maintain the quality of your biosafety reviews.
Dedicated Team of Experts
Navigate complex regulatory requirements with our biosafety team and leverage their combined 200+ years of experience in all areas of gene transfer research to advance your study.
Maintain High-Quality Reviews
Save time without sacrificing the quality of biosafety review. WCG’s IBC is ISO 9001 certified, underscoring our commitment to the highest standards.
Realize the Benefits of a Combined IRB+ and IBC+ Review Process
Increase your time savings and reduce burden on your sites by combining WCG’s IBC+ and WCG’s IRB+. When WCG administers IRB+ and IBC+, we conduct reviews simultaneously and work together so you get maximum efficiency. Learn more about IRB+ today.
Accelerate your gene transfer study today.
WCG’s innovative approach to IBC operational processes provide the fastest turnaround without sacrificing the sanctity of biosafety review. Experience the WCG difference starting with a free IBC+ review consultation. We’re here to help you reach study start-up, faster.