ClinSphere™ Total Feasibility
Optimize and simplify your study planning, site identification, and selection processes.
Successfully address your most critical study planning decision points with WCG’s Total Feasibility solution. Powered by the WCG ClinSphere™ technology platform, this solution delivers:
Clinical Development Insights to support your studies and explore the competitive landscape by comparing clinical trials with similar attributes, using custom data and analytics to support country identification and enrollment modeling.
Site Identification to determine the high-performing, best-fit investigators for your study, leveraging WCG’s proprietary investigator match scores.
Site Feasibility Assessment to optimize the site selection process through strategic outreach with real-time tracking, scoring, and analysis of responses to yield faster decision points. Leverage a self-serve application or choose a high-touch service-oriented experience.
Streamline site feasibility with efficient communication, consistent questionnaires, and robust data intelligence.
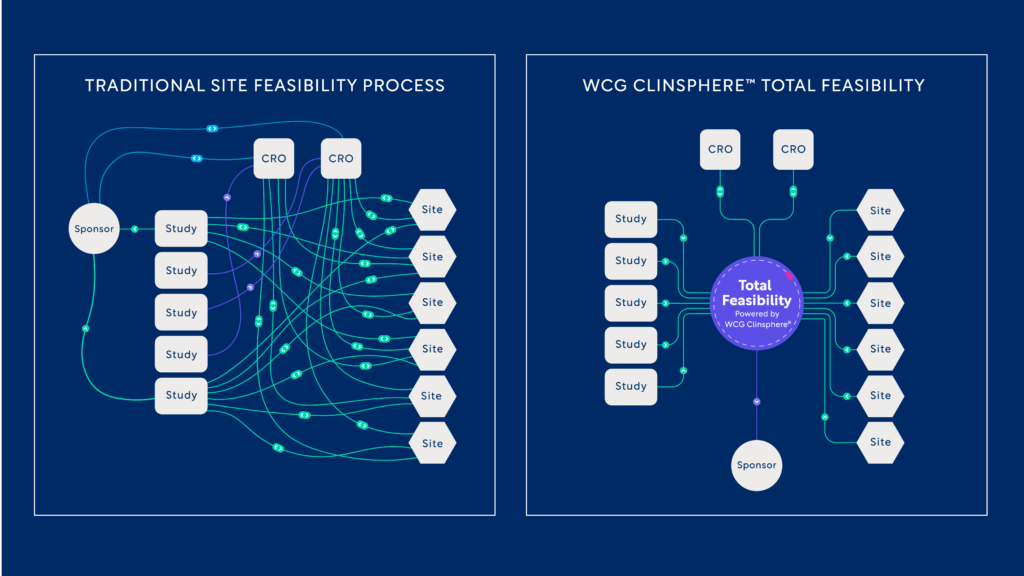
The traditional site feasibility process involves hundreds of touchpoints between sponsors, CROs, and sites for each individual study, causing a high volume of cumbersome and redundant communication. WCG ClinSphere™ Total Feasibility centralizes and standardizes critical study data for sponsors to reduce redundancy and optimize site feasibility and selection activities.
Recent enhancements to the Site Feasibility Assessment capability have been meticulously designed to alleviate the burdens faced by sites and investigators.
Key features include:
Deep Intelligence
Drill down into specific protocol attributes for more actionable insights beyond therapeutic area and indication.
Configurability
Enable rapid development of clinical study questionnaires, and more precise information matched to your study.
Advanced Reporting Capabilities
Unlock new data from surveys for real-time insights.
According to recent research by the Tufts Center for the Study of Drug Development, an average of 200 hours are spent each month per investigative site completing feasibility assessments and site qualification visits for FDA regulated industry-funded clinical trials. Implementing efficiencies allows maximized time for focus on patient care and recruitment.
This next-generation capability reduces the burden on sites and investigators by:
- Streamlining questionnaires for the collection of consistent data points.
- Offering a user-friendly interface for sites.
- Providing sponsors with interactive dashboards for progress and status updates.
By the Numbers
WCG’s Total Feasibility solution provides consistent responses around the globe to deliver quality data for your study
response rate
double the industry average
countries with sites responding
ensuring a truly global impact
questionnaires completed
and counting
Drive deeper and more productive site relationships with WCG ClinSphere™ Total Feasibility
WCG Total Feasibility is powered by ClinSphereTM, an interoperable technology platform for running clinical trials end to end.
Schedule a meeting on WCG’s Total Feasibility Solution
Learn how WCG’s Total Feasibility helps you achieve extraordinary accuracy and cooperation from leading sites—ensuring faster qualification and a smoother, more productive relationship throughout the study. By completing the form, someone will get back to you within 24 hours to schedule a meeting.