eCOA/ePRO Platform
Enhance signal detection and reduce your clinical trial error rate with WCG’s leading eCOA/ePRO Platform
The high and costly failure rate of clinical trials is due in large part to imprecise endpoint measurements that add noise and impair signal detection.
Our platform combines cohesive, interoperable electronic Clinical Outcomes Assessment (eCOA) software and accurate, expert clinician services to improve the collection, management, and analysis of clinical study data. This results in a dramatic improvement in clinical assessment accuracy and a smoother, more direct path to market.
Let’s discuss strategies for improving your clinician- and participant-reported data:

Pair clinical expertise with innovative technology to achieve greater accuracy
Our expert, calibrated raters administer live assessment of trial participants at sites or remotely to improve study integrity and minimize bias and variability.
Our training experts design participant and study staff training to improve the accuracy of your therapeutic findings.
Our global cohort of expert clinicians review assessment data and recordings. They provide insight into study progress and flag potential discrepancies and surface quality issues.
Our statisticians and clinical scientists help build data quality metrics that allow study managers to assess study progress.
Our platform tracks all clinician-reported, observer-reported, and participant-reported outcomes. This results in standardized assessments, more accurate signal detection, and more reliable data quality.

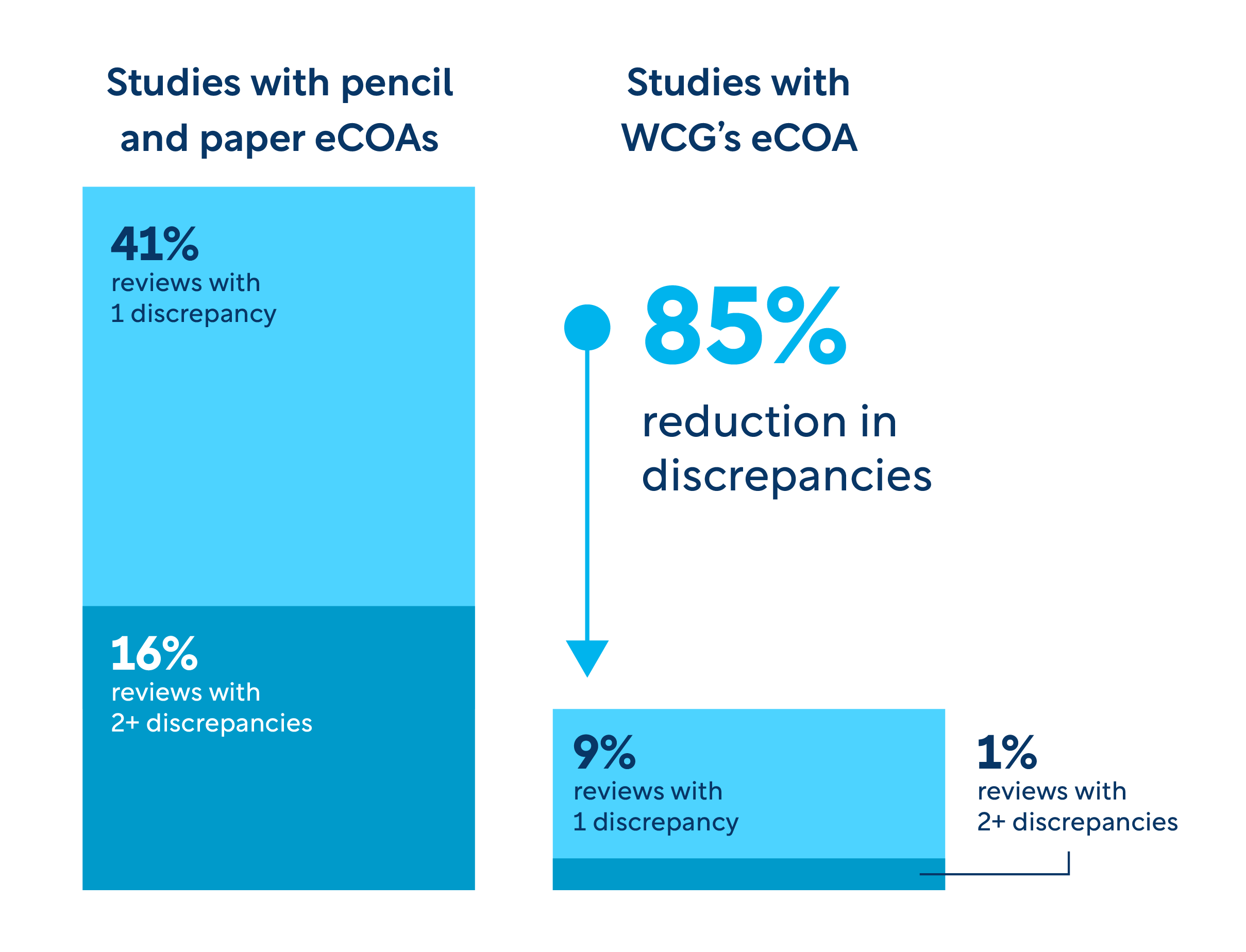
The power of WCG’s science-driven technology
Smart scientific design: Measurement science and eCOA expertise are applied to the design and configuration of your solution.
Overall improved data quality: Our unique form-builder technology incorporates edit checks and clinical guidance to ensure the data captured in the assessments is accurate. Our remote data capture service for participants and caregivers delivers immediate item-by-item uploads to our database, minimizing risk of data loss.
Built with participants, for participants
Participant Adherence: We create study solutions with a deep understanding of measurement science and functional design to help ensure questionnaires are completed to minimize missing entries. Participants complete questionnaires as required through use of reminders and alerts, while date/time stamping documents protocol adherence.
Participant Engagement: Participant are engaged throughout the study through messaging, availability of additional training, and notifications of study visits and study information. Real-time monitoring of participants is enabled by sites through our eCOA/ePRO platform to view compliance, messages from participants and participant status.
Rapid setup and easy deployment
Quick development times: We leverage our proprietary form-builder technology to enable views of the system as early as the kick-off meetings with sponsors.
Ease of enrollment: Avoid problems associated with manual entry of study/participant numbers into devices through the use of QR code technology.
To see WCG’s eCOA/ePRO platform in action, schedule a demo and consultation today
The eCOA/ePRO platform–along with our complete suite of clinical services–make WCG the most proficient and reliable global partner for improved outcome measurement and smarter, faster, and more successful clinical trials.