In today’s challenging drug development landscape, clinical research sites often struggle with the completeness of budgets. Needs include covering costs, reinvesting in the assets needed to optimize research – such as technology – and responding promptly. In this two-part blog, Part I will explore current industry trends, then discuss questions around best practices for improving the process, and Part II will answer questions regarding sponsor/CRO roles, fair market value, and technology use.
Negotiation Timelines
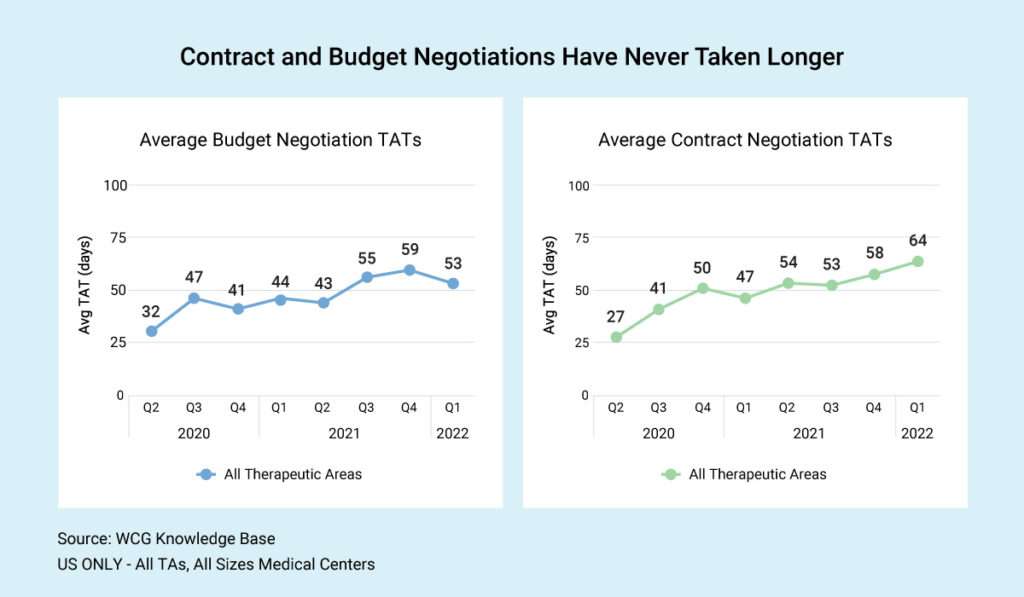
Industry-wide, US sites report an average of 52.5 days from the time the budget comes in until budget finalization. The average contract timeline, including budget negotiation, is about 63.8 days. As you can see in our graphic, budget and contract negotiations have never taken longer.
What is the outcome of this time and effort? Often, we focus on cost per patient or the individual patient budget in negotiation, but let’s look at how many patients are being billed.
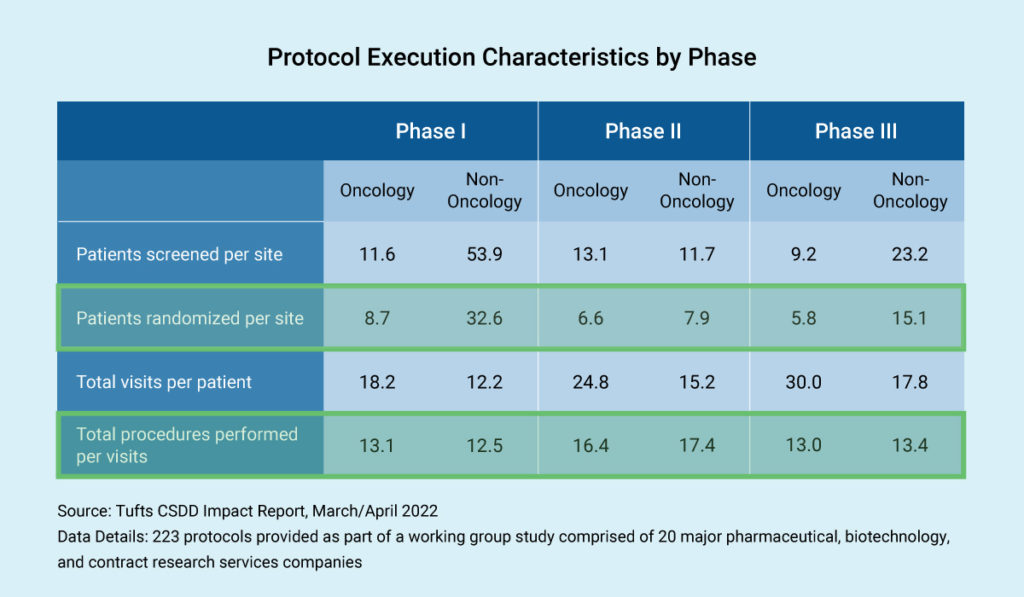
Trial complexity and budget negotiation are correlated. As expected, in non-oncology therapeutic areas, we see the highest number of patients in Phase I trials, where they are not being randomized. In Phase III trials, we also see non-oncology having many more patients than oncology trials.
Next, let’s consider the total number of procedures performed per visit. Study protocols can involve tremendous detail, which affects the budget. Imagine a study involving 20 or 30 visits, requiring massive endeavors to understand these procedures in budgetary terms.
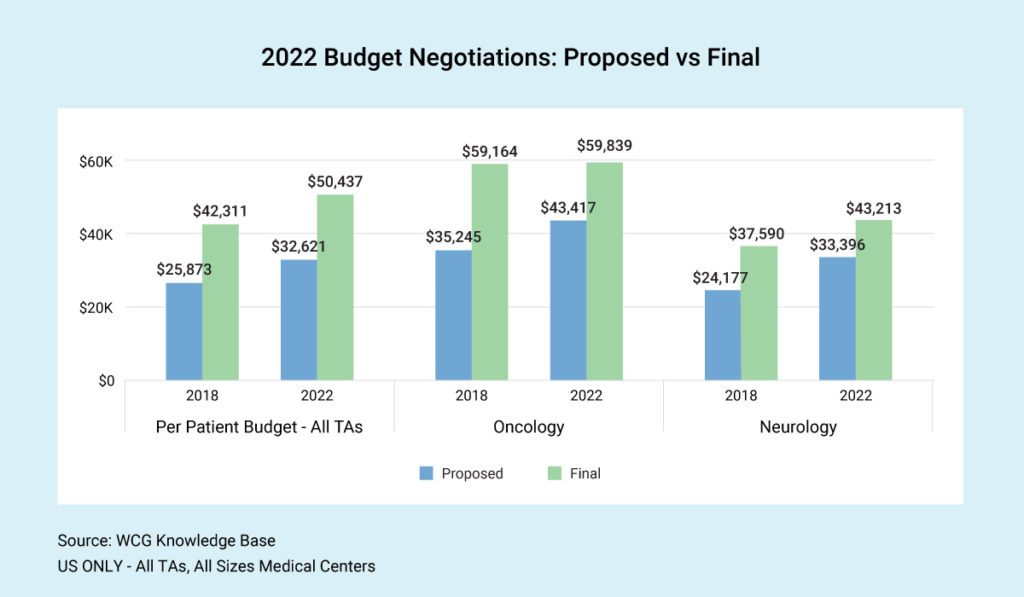
It’s no secret that clinical trial budgets have increased in recent years. However, the spread between proposed and final budgets is about the same. Across therapeutic areas, we see final budgets today coming in more than 50% higher than at the start of budget negotiation. This outcome begs the question, “Should sponsors start higher?” Is the time spent on negotiation worth the squeeze and the amount of money saved on the back end?
Given this background, let’s explore several important questions that clinical research sites are asking:
Question 1: How can sites improve budget negotiation timelines and accelerate study startup?
A master fee schedule between the sponsor and the site can improve activation times. It should address standard administrative fees and include language regarding any future fees. Remember to adjust for inflation; the chargemaster will typically increase by 5% annually.
How can we enhance communication and decrease counteroffers to make the negotiation process more efficient? Timelines are crucial, especially for highly competitive trials, so make your negotiation timetable clear from the start. Include all available documentation for administrative and procedural costs, as many sponsors request this.
Finally, remember the correlation between coverage analysis and budgeting. Many sites enter negotiations with a budget counteroffer based on a Medicare coverage analysis to ensure compliance. Sponsors may not be aware of Medicare billing rules, so communicate that your budget is based on a coverage analysis that includes these rules. Sponsors may even reimburse you for the time and effort involved.
Question 2: What are some best practices for standardizing internal budgeting processes?
When estimating investigator and staff time, enter negotiations with your site’s documented hourly rates for PI, coordinator, and nurse time – including salary and benefits. Offer the sponsor a breakdown of how long key processes take, such as informed consent and adverse event (AE) reporting. When facing multiple procedures during each patient visit, the average hourly rate will help determine the time and effort for each study participant.
Next, let’s consider how sites can increase procedural costs from the initial sponsor offer. First, ensure that you and the sponsor are on the same page regarding procedures and that the sponsor has the correct CPT code. Provide documentation (typically signed PDFs), so the sponsor can see the standard rate your site requests for all clinical trials based on your research chargemaster.
Inflation is an important consideration. Take the length of the study in years and inflate today’s cost for a procedure by 5% annually, using the average. Regarding screening fees, some sites are negotiating a flat pre-screening fee. Why? It can be difficult to determine exact fees for screening when therapeutic areas such as oncology pre-screen every patient.
Overhead (indirect cost) is the rate applied to every research study to cover building use, maintenance, IT, software and infrastructure, technical support, and related costs. The indirect rate applies to all budgetary items as well as pass-through items. Have documentation available, apply overhead consistently across all studies, and stand firm with sponsors.
Finally, how can sites cover computer program/technology training and implementation? This question is a hot topic, as technology-related activities consume staff time and are not covered adequately in most site budgets. One angle is to incorporate a sponsor-specific technical fee associated with initiation visits.
What’s next?
See Part II in this series, where we answer questions regarding sponsor/CRO roles, fair market value, and technology use. We also address budgetary considerations for diversity, equity, and inclusion.
Do you need help handling your clinical trial budgeting and negotiation? Connect with WCG today.
Accelerate your start-up timelines and enroll patients sooner with WCG's Study Start-up solutions
Complete the form to schedule a consultation with WCG.
